‘Digital health’ increasingly surrounds us in the clinical environment, although it is not always embraced among all healthcare professionals. Modern technologies and digital appliances have a significant impact on the way we care for patients by offering innovative ways to converge technology, connectivity and people. This should translate into improved care and clinical outcomes.1 The term is often used more broadly to include ‘e-health’, as well as developing areas, such as the use of advanced computer sciences in the fields of big data, genomics and artificial intelligence.
The WHO has described e-health as the cost-effective and secure use of information and communication technologies in support of health and health-related fields, including healthcare services, health surveillance, health literature, health education, knowledge and research.2 Currently, there is a need to adopt evidence-based practice via research with regard to digital health solutions. This can facilitate reimbursement, promote their use on a wider scale and make them more readily available.
In the US, 53% of individuals over the age of 65 years have smartphones and 62% use their smartphone for health enquiries.3,4 It has been estimated that 83% of payers and providers believe that consumers need to take more control of their health in a value-based system.5 Mobile wireless technologies for public health – referred to as m-health – have been shown to increase access to health information, services and skills, as well as promoting positive changes in health behaviours to prevent the onset of acute and chronic diseases.6 It is the responsibility of healthcare practitioners to remain up to date with available options to provide appropriate advice and guidance to our patients.
Device follow-up is a mandatory part of the care for patients treated with cardiac implantable electronic devices (CIEDs). These include permanent pacemakers (PPMs), ICDs, cardiac resynchronisation therapy (CRT) pacemakers and CRT defibrillators and implantable loop recorders. After implantation, CIEDs require close monitoring. This is a highly technical and specialised process requiring dedicated structures, equipment and the interaction of several healthcare personnel. It needs to be done in a timely manner to safely and effectively deliver therapy and is guideline driven.7
There has been a steady increase in the number of patients with CIEDs. In 2016, a total of 547,586 PPMs, 105,730 ICDs and 87,654 CRTs were implanted worldwide in 53 European Society of Cardiology (ESC) member countries. Between 2007 and 2016 there was an increased rate of implantation of various CIEDs in ESC member countries. Implantation rates for PPM increased from 619 per million inhabitants in 2007 to 742 per million inhabitants in 2016, ICDs from 92 per million inhabitants in 2007 to 133 per million in 2016 and CRTs from 53 per million inhabitants in 2007 to 118 per million in 2016.8 This has imposed a significant burden on the already limited resources arising from their follow-up in outpatient clinics. It is estimated that the number of encounters for CIED follow-up is approximately 2.2 million per year in Europe alone. Therefore, there is a need to organise follow-up of patients with CIEDs efficiently and effectively.7
Remote Follow-up and Monitoring of Cardiac Implantable Electronic Devices
Traditional follow-up of CIEDs involved patients attending clinics where their devices were interrogated using a wand-based radiofrequency (RF) system that communicates with a programmer. The frequency of this depends on the device implanted and the patient’s specific clinical condition. For patients with pacemakers this might mean that they would only be seen once a year. Important clinical diagnostics might, therefore, be missed and the opportunity to intervene lost. A good example of relevant clinical data that can be missed (or at least manifest unacceptable delays before reaching the attention of the responsible healthcare practitioner with traditional follow-up) are clinically relevant but asymptomatic episodes of AF that would require timely intervention. This intervention might include assessing the appropriateness of and starting anticoagulation as per guidelines to minimise the risk for thromboembolic events/strokes.
Remote follow-up of CIEDs refers to the process of routine scheduled remote device interrogations, where transmission of data occurs based on pre-specified parameters related to the device functionality and clinical events. These systems usually consist of base units that reside in the patient’s home and communicate with their device either wirelessly or using an RF-based solution. The base unit then transmits the data using either cell services or landline internet connection to the company-specific remote follow-up system. It is structured to mimic conventional in-clinic checks but provides the opportunity for alert-based interactions and more frequent follow-up with monitoring between scheduled transmissions (remote monitoring).9 While more frequent follow-up and more monitoring may result in an increase in the transmitted data, acting on this increasing generation of data should be driven by guidelines to reduce the risk of overuse of remote monitoring. Remote follow-up offers an opportunity to resolve some of the challenges associated with the delivery of effective CIEDs follow-up by improving clinic efficiency and improving the patient’s adherence to follow-up.10–14
Remote care (RC) has proven superior to conventional monitoring in many aspects. RC in pacemaker patients is associated with increased survival, and patients with high RC adherence have shown 53% greater survival than patients with low RC adherence and 140% greater survival compared with no RC.15 The Pacemaker Remote Follow-up Evaluation and Review (PREFER) study highlighted that the diagnosis of clinical actionable events – namely AF, fast ventricular rates in response to atrial arrhythmias, non-sustained ventricular tachycardia (VT), abnormal device or lead parameters and change in percentage of ventricular pacing – was higher and 26% faster in patients with RC.16 Furthermore, the Evaluate the Benefits of Pacemaker Follow-Up With Home-Monitoring (COMPAS) trial demonstrated that there were 66% fewer hospitalisations as a result of atrial arrhythmias and overall 56% fewer ambulatory visits for the remotely monitored pacemaker recipients.12 This all translates into lower healthcare expenditure in office visits in remotely followed-up PPM patients.17
If the system requires the patient to positively interact with the remote system, then this adds the potential to lose adherence. Several randomised trials have shown that RC is more effective in achieving patients’ adherence as well as timely scheduled follow-up goals. Large cohort analyses of databases have consistently shown improved survival rates in patients undergoing RC.18,19 RC represents the new standard of care for patients with CIEDs, with alert-driven inpatient evaluation replacing routine clinic interrogation. This has been reflected in international guidelines recognising RC as a Class 1 recommendation for certain aspects of CIED follow-up, such as lead function, battery management, reduction in inappropriate shocks from ICDs and the early detection of AF.20
Challenges to the Adoption of New Digital Health Solutions in Remote Care
Physicians
Physicians have to provide solutions that ensure the validity and accuracy of the handled data without compromising on its integrity or the quality. Furthermore, any patient data have to be handled securely. This flow of data requires infrastructure changes and revision of traditional workflow and patient pathways. The organisational model needs to be designed to act in a timely fashion to alerts, but recognising that the system does not replace emergency care for unwell patients.
Patients
New digital health solutions in RC need to focus on patients’ enablement, a concept that describes patients’ ability to better understand, or cope with, participate in or have a greater responsibility for their own care.21 Patient education is key to success. In order for the new digital health solutions to work, patients need to have an acceptable degree of health, as well as digital literacy. It is important to take the time to explain to patients the expected reaction times with telemedicine, how to react during emergencies and their responsibilities to keep contact information up to date and maintain the function of the equipment and appropriate communications. New digital health solutions need to be able to promote the shift to exception-based assessments, reducing the economic costs for the patients in order to motivate patients to adhere to follow-up requirements and promote engagement with clinical services.
Application-based Remote Monitoring
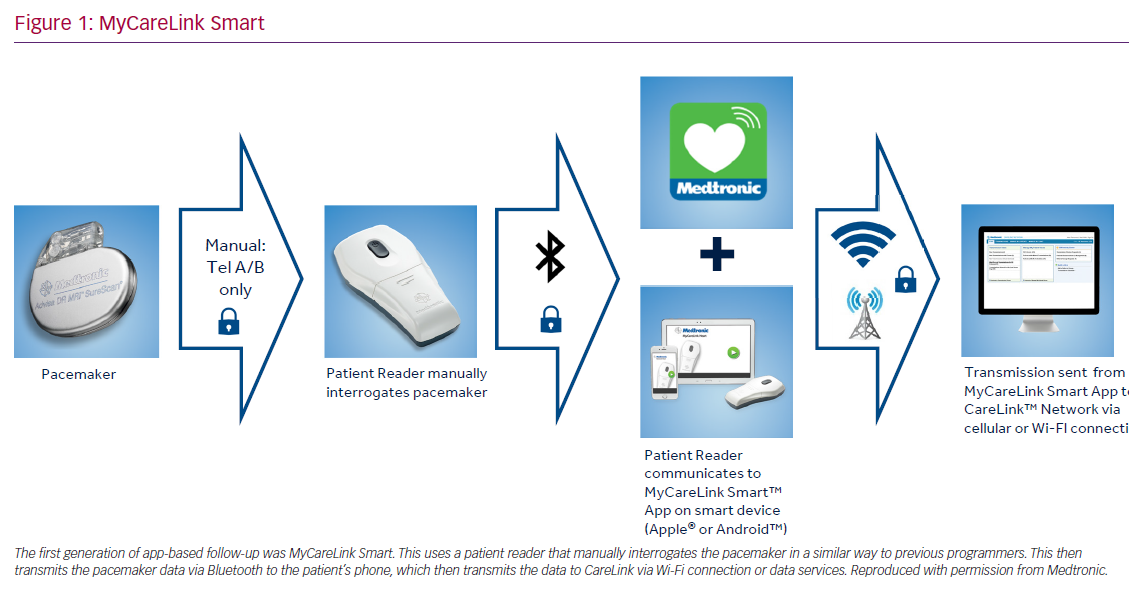
MyCareLink Smart (Medtronic) is a first-generation, application-based remote management system for CIEDs. This is a first of its kind system, with no other comparable applications that will allow for meaningful comparison. Patients who have application-based, RC-enabled CIEDs are given a hand-held reader device and are instructed to download a compatible application on their smartphone/tablet.
The RC process involves turning on and placing the reader over the implanted device manually so that the device can be interrogated and data retrieved. The reader then transmits the information via Bluetooth to the application on the patient’s smartphone or tablet, which then sends the information through to the CareLink network via cellular or landline internet connection. The information is then available to the clinical team in the usual manner. This system replaces the conventional transmission unit with a reader and the patient’s smartphone/tablet. The process requires the patient to interrogate their device with the reader. Patients are given a timetable of when to interrogate the device so that the data can be transmitted to the CareLink network (Figure 1).
A large retrospective analysis was performed in the US of more than 95,000 patients who were enrolled in the CareLink database, using MyCareLink Smart. The analysis looked into the proportion of patients who adhered to the follow-up transmissions according to the clinic schedule. There were 48,016 patients assigned app-based remote follow-up, and 40,511 (84.4%) of them activated their devices for RC. Adherence analysis was limited to 14,232 patients who activated their RC and had at least 12 months of follow-up after activation. Of these patients, 89% were considered adherent, as per Heart Rhythm Society guidelines, as they had at least one more transmission within 3 months to 1 year after activation. There was no difference in adherence to follow-up in patients having a generator change or a de novo device. There was also no difference between men and women. The high percentages of adherence across all age groups suggest patients’ ability and desire to continue using RC.22
A further retrospective analysis was carried out on 156,426 patients in the US who were enrolled using the CareLink System between January 2012 and December 2016. The aim of the analysis was to assess patients’ compliance to scheduled transmissions in a real-world setting among different age groups. Over a mean follow-up of 3 years, compliance to scheduled remote monitoring since activation was 61.8% and sub-group analysis identified patients ≤60 years old (52.8%; 95% CI [52.1–53.5]) to be less compliant than patients >60 years (62.8%; 95% [CI 62.6–63.1%]). The outcome of this analysis is that less than two-thirds of patients are adherent to the follow-up regime at 3 years. This is clinically unacceptable in terms of comprehensive CIED follow-up. The system requires timely manual transmissions using the patient reader and so requires active engagement over the life of the patient and device. It is probable that this is the stage of the pathway that results in reduced engagement because it requires active action from the patient. With this in mind, increasing the automaticity of the system would likely result in more optimal follow-up adherence.23
Next-generation Application-based Remote Monitoring
The latest platform of pacemakers developed by Medtronic uses Bluetooth Low Energy to communicate with its programmer. Bluetooth Low Energy is different to the Bluetooth used in household items, such as speakers and hands-free headsets. These devices require high amounts of data transfer at fast rates. Bluetooth Low Energy is slower and for low volumes of data, so is perfectly suited to CIED interrogation. The current drain on the device is, as a consequence, approximately two-thirds of current energy drain used in historic device communication. As most current smartphones/tablets use Bluetooth for communication, this provides the perfect partnership.
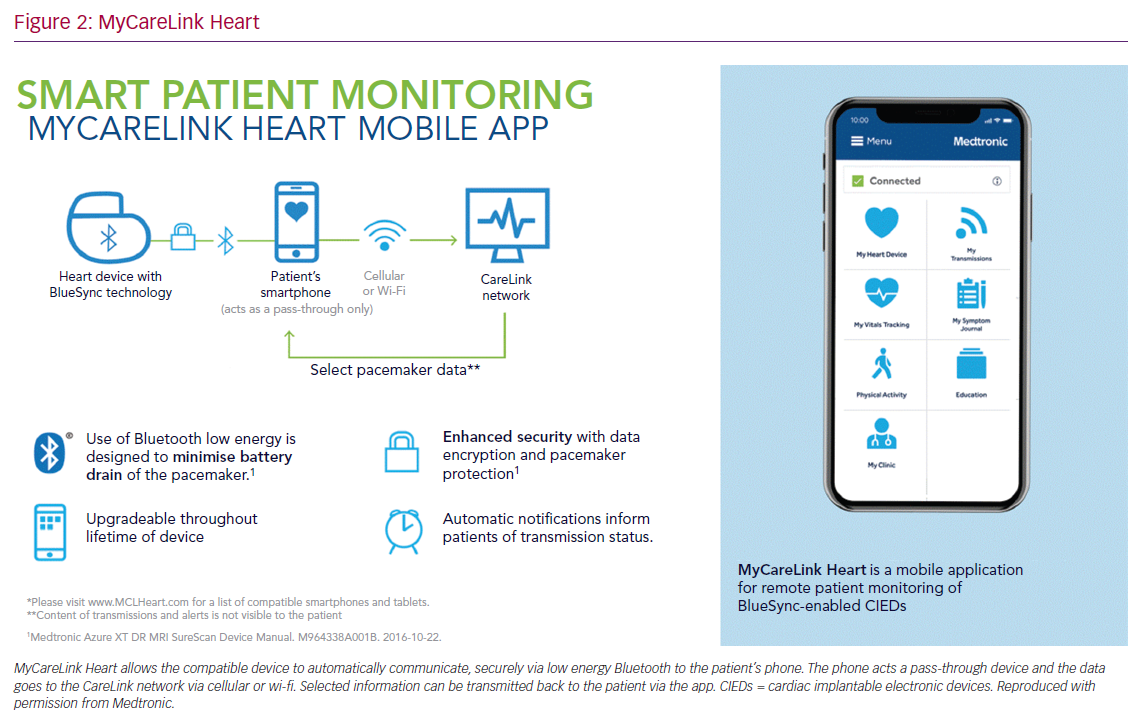
MyCareLink Heart is a fully automated app-based RC system that communicates with Medtronic pacemakers using Bluetooth Low Energy. The compatible CIED is paired directly and automatically to the patient’s smartphone/tablet via the application. The patient’s device then transmits the data to CareLink securely via cellular network or landline internet connection. It uses the patient’s phone/tablet as a ‘pass-through’ device with no data being retained on the phone/tablet. Select data can then be passed back to the patient’s device via CareLink and is then visible on the MyCareLink Heart app (Figure 2).
This system requires the application to be open in the background on the patient’s device so that communication can occur, allowing passive data transfer, in contrast to active data transfer that requires action from the patient. The schedule of transmissions is directed by the clinic through the CareLink network. If a schedule is due and the patient does not have the application open, then a push notification will be sent to prompt action from the patient.
Patients’ interaction with the application provides a platform to prompt their consent on both passive and active transfer of data on an ongoing basis. Device and clinical alerts can be programmed on, so if there are any alerts the physician will receive notification of these outside of the usual follow-up regimen, with regular connectivity between the CIED and the patient’s device. These alerts include events related to lead impedance, low battery voltage, atrial tachycardia (AT)/AF burden, VT episodes, fast V rate during AT/AF, capture management and percentage V pacing.
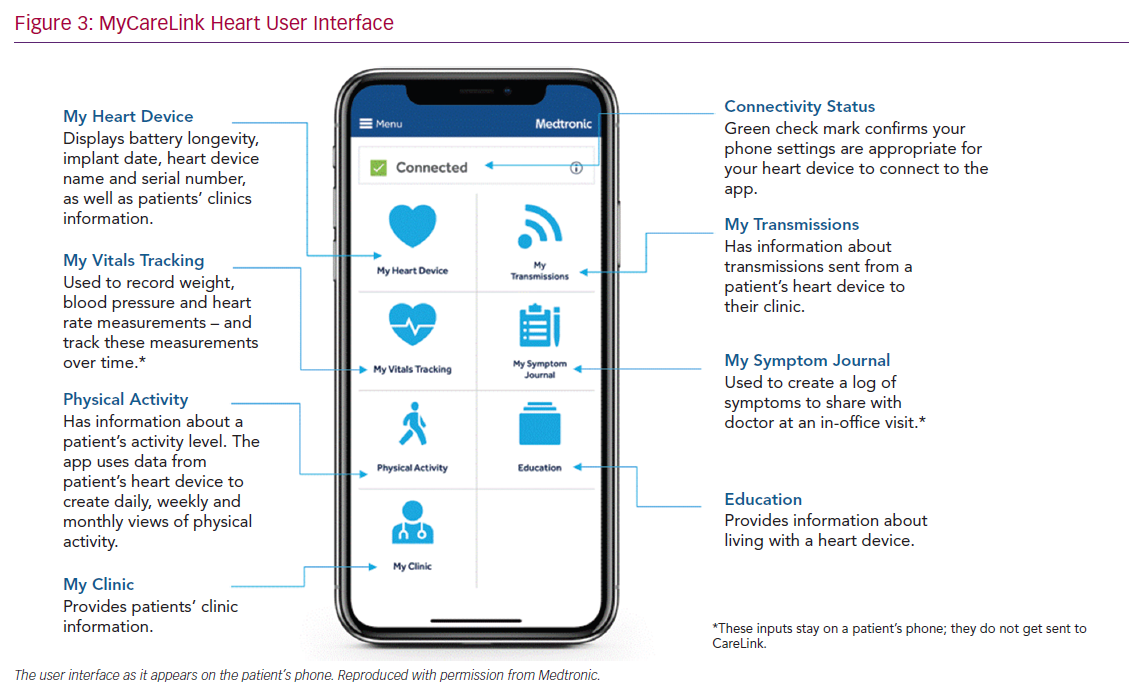
The system has the facility for patients to make an additional transmission if required. To ensure that this facility is not used inappropriately the patient is prompted with “Does your clinic know that you are going to send a transmission?”. Limited data are fed back to the MyCareLink Heart app, including battery longevity, activity levels determined by the accelerometer and the status of the patient’s transmission schedule. The application also allows patients to record vital signs and any symptoms on a daily basis. The data are not transmitted to CareLink but allow a record that the patient can show to their healthcare professional. There are education signposts within the application and also essential details about the patient’s device and leads that can act as their CIED identification card (Figure 3).
This next generation of app-based monitoring represents an example of Quantifying Self Hybrid Models (QSHMs), which combine features of patient reported outcomes measures (PROMs) and objective telemonitoring into a system that integrates passive data collection and active patient reporting. They help to overcome the unreliable subjectivity of PROMs and the absolute objectivity of telemonitoring alone. The use of QSHMs promotes patients’ enablement, and initial studies evaluating the impact of QSHMs in chronic disease management have been promising.24
A prospective randomised control trial conducted in Toronto, Canada, examined this in 110 patients with diabetes and uncontrolled systolic hypertension. Patients in the intervention group were provided with a home blood pressure (BP) telemonitoring system that provided self-care messages on the patients’ smartphones based on the averages of the transmitted readings. Patients in the control group were provided with a home BP monitoring system without the self-care messages. At 12 months, there was a significant reduction in the mean daytime systolic BP (−9.1 mmHg and −1.5 mmHg), and mean daytime diastolic BP (−4.6 mmHg and −1.3 mmHg) in the intervention group compared with the control group, respectively. In addition, 51% of the patients in the intervention group achieved the guideline recommended target BP in comparison to 31% of the control group. There were no significant changes in the number or classes of antihypertensive medications at exit and there was no difference in the number of physicians’ office visits between both groups.25
Expectations and Challenges
Patients’ expectations from this technology would be to provide them with more information on the status of their device, in particular battery longevity and status of the scheduled transmissions, which is fed back to them through the application.
Physicians’ expectations from this new technology would be to provide them with concise, clinically relevant information, promoting efficiency. The main concern would be overuse of this technology because of the increased generation of data. Initial experience with this next-generation application showed that after the introduction of notifications prompting patients to acknowledge unscheduled downloads and asking them if their healthcare practitioner was aware of them, most of the time they don’t go through with them, making a high volume of unscheduled downloads by the patients less likely. Another concern that would need to be addressed is healthcare practitioners’ responsibility. With the increased automaticity of the follow-up process with minimal – if any – action required by the patient, it is implied that more responsibility for the device follow-up is shifted from the patient to the healthcare practitioner/physician. This means that guidelines and pathways need to be implemented to react in timely manner to the downloaded data. It is also important to stress the fact that the system does not replace emergency care for unwell patients.
This brings another challenge – the time and the cost of patients’ education. It is important to take the time to explain to patients the expected reaction times with this new system. Furthermore, they also need to understand their responsibilities within this new system such as maintaining the function of the equipment and appropriate communications and what to do in case of an emergency. In practice, if implementation of this system proves to promote the shift to the more efficient exception-based follow-up, this would not only reduce the economic cost for the patients under follow-up for implanted devices but also would lower healthcare expenditure because of the reduction in the number of office visits needed to be dedicated to these patients. This has the potential to benefit the population as a whole by freeing up specialists outpatient appointments and reducing waiting times.
BlueSync Field Evaluation
BlueSync technology is intended to enable more secure automatic wireless RM via the Medtronic CareLink network with security controls implemented to protect the integrity of the device functionality and protecting patient data. BlueSync field evaluation is an observational prospective study that started recruiting patients in April 2018, with 254 patients enrolled and paired with MyCareLink Heart app. This is a global study (NCT03518658) that includes 104 patients in Europe. The primary objective is to measure the percentage of CareLink quarterly scheduled transmissions successfully completed within a reasonable timeframe. Secondary objectives include patient compliance to pre-scheduled CareLink transmissions, patient adoption to remote monitoring with the new application and patients and healthcare practitioners perceived value and user experiences with the application.
Wireless Programming with BlueSync Technology
The security of data transmission and communication is always a concern in healthcare environments. Programmers are used in the operating environment at the time of CIED implantation and in the clinic if programming adjustments are required. Currently, there are no facilities in place that allow for remote programming of CIEDs. The programming system is comprised of three components with incorporated tamper-proofing protections: a downloadable software application, a proprietary patient connector for secure connectivity and a base unit that communicates with the pacing system analyser. The patient connector and the base unit communicate with the tablet using Bluetooth Classic technology. The patient connector communicates with the implanted device using Bluetooth Low Energy to minimise battery drain of the CIED. The system has rigorous security enhancements with multiple levels of encryption in the device, the programming communicator and the programmer.
Conclusion
There has been a steady increase in the number of patients with CIEDs, imposing a significant burden on the already limited resources arising from their follow-up in CIED outpatient clinics. RC offers an opportunity to resolve some of the challenges associated with the delivery of effective follow-up by improving efficiency and patient engagement.
Database analyses have consistently shown improved survival rates in patients undergoing RC. They now represent the new standard of care replacing routine clinic interrogation. Current systems require a significant degree of patient interaction to ensure that follow-up schedules are maintained. Remote follow-up requires the physical act of the patient interrogating their CIED using a communicator device. The MyCareLink Heart system increases the automaticity of this concept by seamless Bluetooth communication between the patient’s CIED and their smartphone/tablet. Further prospective studies will evaluate the true value to both patients and healthcare professionals.