Until 2021, medical treatment of patients with heart failure (HF) and preserved ejection fraction (HFpEF) was mainly limited to diuretics to improve symptoms of HF, while no therapies demonstrated a mortality/morbidity benefit to these patients.1 True HFpEF has been found to have multiple pathophysiological causes.2 According to the 2021 European guidelines, β-blockers, mineralocorticoid receptor antagonists, renin–angiotensin–aldosterone system inhibitors and angiotensin receptor neprilysin inhibitor (ARNI) might be used for patients with mildly reduced left ventricular ejection fraction ( HFmrEF; 41–49%), but with a IIB class of recommendation.1 Indeed, the PARAGON-HF trial recently showed a trend towards better outcomes in HFpEF patients treated with sacubitril/valsartan (an ARNI) compared to valsartan, especially in the lower end of the left ventricular ejection fraction (LVEF) spectrum, in women, in patients recently hospitalised for HF, and in those with higher high sensitivity troponin values at baseline.3–7 However, while the Food and Drug Administration has approved sacubitril/valsartan use in patients with ‘below normal’ LVEF, the European Medicines Agency has not.
To accomplish the single greatest unmet need in cardiology, the EMPEROR-Preserved trial has been testing the hypothesis of a beneficial effect of sodium–glucose cotransporter 2 inhibitors (SGLT2I) in HFpEF.8 This trial has been recently published and has shown that SGLT2I empagliflozin can be used as an effective therapy for HFpEF. This drug was able to reduce the combined outcome of hospitalisation for HF (HHF) and cardiovascular (CV) death in patients with HFpEF, compared to placebo. Importantly, the conclusions of this important trial apply to both HFpEF and HFmrEF, since the evidence involved patients with LVEF above and below 50%, and patients with and without diabetes.
In this paper, we provide insights into the rationale for and practical use of SGLT2I in HFpEF patients, using the definition of HFpEF as patients with LVEF >40% according to contemporary HFpEF trials enrolment criteria.
Rationale for SGLT2I Use in HFpEF
SGLT2I Mechanism of Action
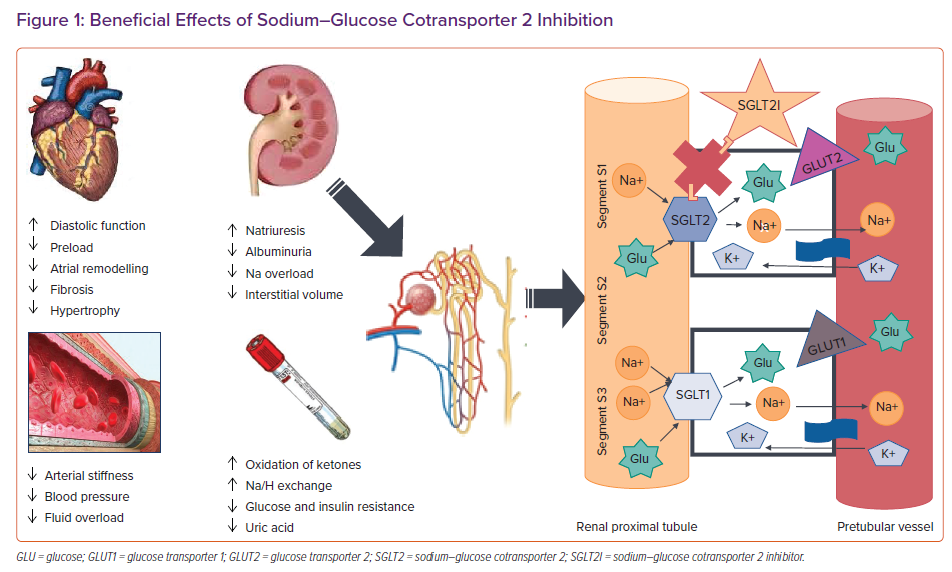
SGLT2 proteins are mainly expressed in the proximal convoluted tubule of the kidneys and are responsible for reabsorption of roughly 90% of filtered glucose together with sodium, making it the ideal target to reduce blood glucose levels using glycosuria in people with diabetes. Thus, a new class of drug was developed to inhibit SGLT2, which includes dapagliflozin, empagliflozin and canagliflozin, while another compound, sotagliflozin, inhibits both renal SGLT2 and intestinal SGLT1. Inhibiting SGLT2 causes a lower threshold for glycosuria from the usual glycaemia value of 180 mg/dl to as low as 40 mg/dl. Importantly, people with genetically non-functional SGLT2 and severe glycosuria are usually healthy, with a low risk of hypotension and hypoglycaemia, suggesting that the use of SGLT2I is safe even in patients who do not have diabetes.
In addition to reducing HbA1c levels in patients with type 2 diabetes, SGLT2I have a significant effect on natriuresis and osmotic diuresis.9,10 However, unlike diuretics, they do not deplete intravascular volume, but instead reduce interstitial volume.11 SGLT2I are associated with reductions in blood pressure of about 3–5 mmHg, without an increase in heart rate.12,13 Moreover, other mechanisms could also be involved, such as a reduction in arterial stiffness.14 Importantly, the urinary glucose excretion caused by SGLT2I leads to a loss of calories and most of the studies have consistently shown weight loss of 2–3 kg when they are used.15 Therefore, SGLT2I have favourable effects on diabetes, hypertension and overweight/obesity, which in turn have a significant impact on left ventricular (LV) diastolic function. It has also been suggested that SGLT2I may improve cardiac metabolism and bioenergetics, shifting metabolism towards the oxidation of ketone bodies, which has been shown to be associated with myocardial benefits.16 Furthermore, SGLT2I seem to play a role in ion exchange, since downstream inhibition of myocardial Na+/H+ exchange has been shown to lead to lower levels of sodium and lower levels of inhibition of calcium in cardiomyocytes, which improves contractility and mitochondrial function (Figure 1).17
Data on SGLT2I and Diastolic Dysfunction
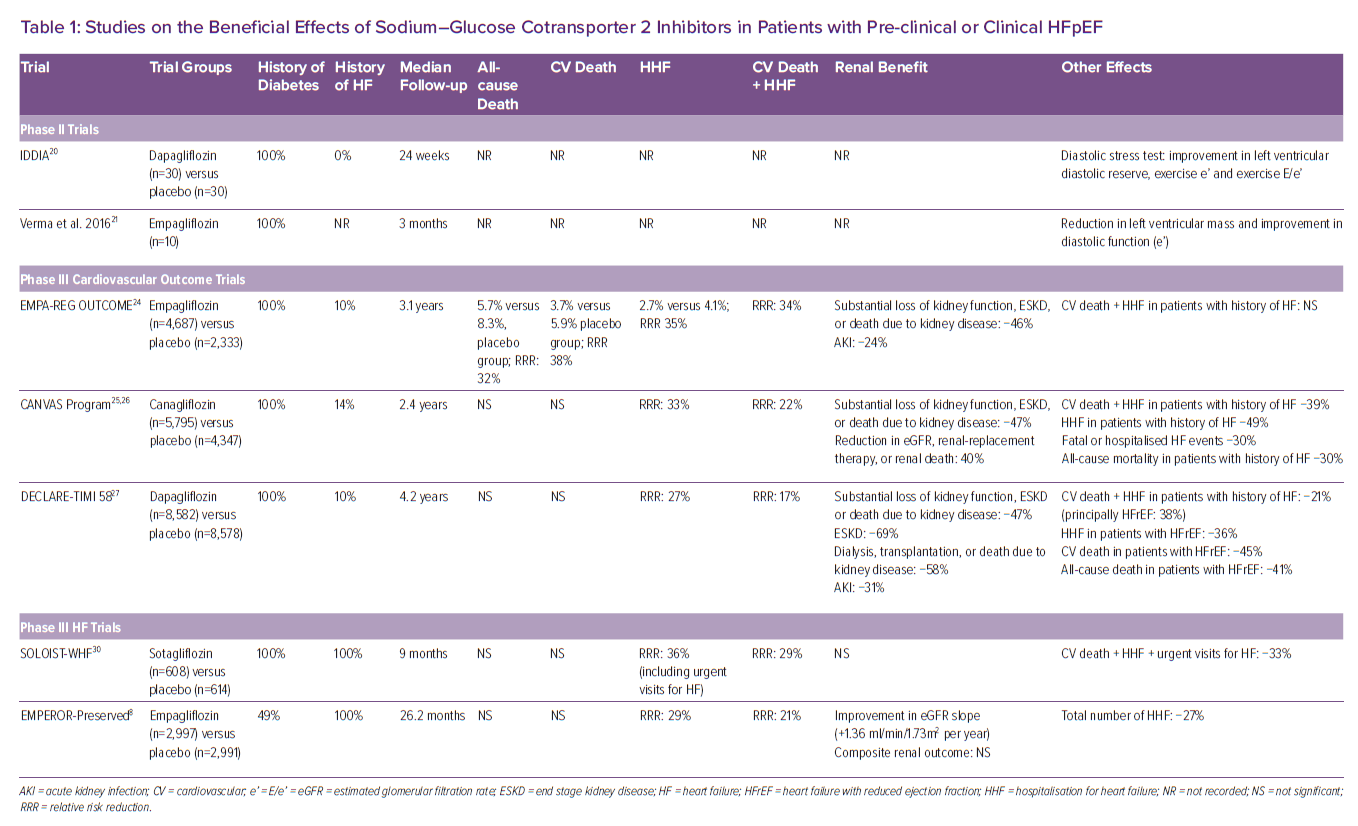
In 2018 it was shown that empagliflozin is able to improve diastolic function in anaesthetised rats and in isolated human ventricular trabeculae.18 Furthermore, the SGLT2I empagliflozin reduced LV mass, improving both wall stress and diastolic function in a rodent model of HFpEF.19 This was followed by the observation that dapagliflozin improved ventricular diastolic dysfunction in patients with diabetes.20 In a small study of patients with type 2 diabetes and established cardiovascular disease, Verma et al. reported a significant reduction in LV mass index and improved LV diastolic function after 3 months of SGLT2I initiation (Table 1).21
Data on Diabetes/Cardiovascular Outcome Trials
All the aforementioned mechanisms of action provided the rationale to test the cardiovascular benefits of SGLT2I, first in patients with diabetes. The effect of this class of drugs has been successfully tested in different trials that looked at diabetes prevention (EMPA-REG OUTCOME, CANVAS, CREDENCE and DECLARE-TIMI 58), showing a reduced incidence of HF, cardiac events, and the preservation of renal function with the use of SGLT2I (Table 1).22–27 Importantly, despite these previous reports that SGLT2I reduce the risk of HHF in patients with type 2 diabetes, in these earlier trials most patients did not have HF at the time of enrolment. Post hoc characterisation of the HF phenotype, either at the time of randomisation or at the onset of a post-randomisation HF event, suggested that, not only patients with HF and reduced ejection fraction (HFrEF), but also patients with HFpEF might have benefited from treatment, but these analyses had a small number of events and substantial missing data.28,29 Thus, more data were needed in patients with prevalent HF.
SOLOIST-WHF
The SOLOIST-WHF trial was conducted in patients with diabetes and acute HF. A total of 1,222 patients underwent randomisation to the SGLT1 and 2 inhibitor sotagliflozin or placebo and were followed for a median of 9 months. The primary endpoint was the total number of cases of CV death and HHF and urgent visits for HF (first and subsequent events). A total of 600 primary endpoint events occurred (245 in the sotagliflozin group and 355 in the placebo group). The rate (the number of events per 100 patient years) of primary endpoint events was lower in the sotagliflozin group than in the placebo group (51.0 versus 76.3; HR 0.67; 95% CI [0.52–0.85] p<0.001). Diarrhoea was more common with sotagliflozin than with placebo (6.1 versus 3.4%), as was severe hypoglycaemia (1.5 versus 0.3%). Sotagliflozin was associated with a significant reduction in CV death, HHF and urgent HF visits even in a subgroup of patients with HFpEF.30 However, the number of events was too small to allow for a reliable estimate of a treatment effect.
EMPULSE
The EMPULSE trial was a double-blind trial conducted in 530 patients with a primary diagnosis of acute de novo or decompensated chronic HF, either with preserved or reduced LVEF.31 Patients were randomly assigned in-hospital when clinically stable (median time from hospital admission to randomisation was 3 days) to receive empagliflozin 10 mg once daily or placebo. Follow-up time was 90 days. The primary outcome of the trial was clinical benefit defined as a hierarchical composite of death from any cause, number of HF events and time to first HF event, or a 5 point or greater difference in change from baseline in the Kansas City cardiomyopathy Questionnaire Total Symptom Score (KCCQ-CS) at 90 days, as assessed using a win ratio. More patients treated with empagliflozin had clinical benefit compared with placebo (stratified win ratio, 1.36; 95% CI [1.09–1.68]; p=0.0054), meeting the primary endpoint. Clinical benefit was observed for both acute de novo and decompensated chronic HF and was observed regardless of LVEF or the presence or absence of diabetes. Empagliflozin was found to be well tolerated.
PRESERVED-HF
The PRESERVED-HF trial was a multicentre, randomised trial of patients with HFpEF designed to evaluate the impact of the SGLT2I dapagliflozin on patient-reported symptoms, physical limitations, and exercise function.32 Globally, 324 patients were randomised to dapagliflozin or placebo. Dapagliflozin improved KCCQ-CS (p=0.001), meeting the predefined primary endpoint, due to improvements in both KCCQ total symptom score (KCCQ-TS) (5.8 points; 95% CI [2.0–9.6]; p=0.003) and physical limitations scores (5.3 points; 95% CI [0.7–10.0]; p=0.026). Dapagliflozin also improved 6-minute walk test (6MWT) (mean effect size 20.1 m; 95% CI [5.6–34.7]; p=0.007), KCCQ-OS (4.5 points; 95% CI [1.1–7.8]; p=0.009), proportion of participants with five-point or greater improvements in KCCQ-OS (OR 1.73; 95% CI [1.05–2.85]; p=0.03) and reduced weight (mean effect size 0.72 kg; 95% CI [0.01–1.42]; p=0.046). Adverse events were similar between dapagliflozin and placebo.
EMPEROR-Preserved
EMPEROR-Preserved was a double-blind trial comparing the SGLT2I empagliflozin versus placebo in patients with HFpEF, which was defined as LVEF >40%.8 In the trial, 5,988 patients with New York Heart Association class II–IV HF were randomly assigned to receive empagliflozin (10 mg once daily) or placebo, in addition to usual therapy. The trial was designed and powered to address three endpoints: CV death or HHF; total HHF (first and recurrent); and renal function preservation, measured as changes in estimated glomerular filtration rate (eGFR) slope. Over a median of 26.2 months, a primary outcome event occurred in 415 of 2,997 patients (13.8%) in the empagliflozin group and in 511 of 2,991 patients (17.1%) in the placebo group (HR 0.79; 95% CI [0.69–0.90]; p<0.001). This effect was mainly related to a lower risk of HHF in the empagliflozin group, with a 28% relative risk reduction. The effects of empagliflozin appeared consistent in patients with or without diabetes. The eGFR slope was significantly preserved, with a mean change of 1.36 ml/min/1.73 m2/year.
Overall, the trial achieved all three primary goals. However, while there was a 9% directional benefit in the reduction of CV death, it did not achieve statistical significance. Concerning subgroups analyses, for the primary endpoint, there was no heterogeneity on the basis of sex, or LVEF above or below 50%. Almost a third split between EF 40–50%, 50–60% and >60%, and the interaction p-value was 0.21 and the HR was <1 for all three groups. Finally, empagliflozin treatment was found to be generally safe although uncomplicated genital and urinary tract infections and hypotension were reported more frequently with its use.
EMPEROR-Pooled
EMPEROR-Pooled was a pooled analysis of two randomised trials, EMPEROR-Reduced and EMPEROR-Preserved.33 A total of 9,718 patients were included in this analysis. The evaluation demonstrated that empagliflozin reduced the risk of HHF to a similar degree – about 30% risk reduction – in EMPEROR-Preserved and in EMPEROR-Reduced. The magnitude of the effect on HHF was similar across a broad range of LVEF below 65% with attenuation of the drug effect at higher LVEF (65% or greater). The analysis also found that empagliflozin reduced the risk of major renal outcomes in EMPEROR-Reduced, but not in EMPEROR-Preserved. However, in EMPEROR-Preserved, when renal outcomes were defined using more stringent criteria, pre-treatment ejection fraction influenced the effect of empagliflozin on renal outcomes in a manner that paralleled the drug’s effect on HHF.34
Tips and Tricks for SGLT2I Management
SGLT2I are safe, well-tolerated drugs. They do not cause hypotension or electrolyte imbalance and they have a diuretic and natriuretic effect, targeting the proximal convoluted tubule and working synergistically with loop diuretics. After an initial decline in eGFR, they are protective of renal function. They do not cause hypoglycaemia, even in patients without diabetes, and they do not need progressive up-titration. These important characteristics explain why they have been named the ‘smartest diuretics’.35 However there are some caveats when using them.
Estimated Glomerular Filtration Rate
In the EMPEROR-Reduced study, there was a transient minimal decline in eGFR immediately after starting SGLT2I. Interestingly, a meta-analysis compared renal effects of SGLT2I and dipeptidyl peptidase-4 (DPP4)-inhibitors showing no eGFR drop in higher eGFR strata, with an immediate reduction in eGFR after SGLT2I initiation only in the lower eGFR strata (<45 ml/min/1.73 m2).22 This event was not accompanied by serious acute kidney injury (AKI)/renal adverse events or hospitalisation. On the contrary, the risk of hospitalisation for AKI was reduced. The medium-term results at 30 and 90 days and the long-term results, however, were strikingly in favour of SGLT2I therapy.
The EMPEROR-Reduced and EMPEROR-Preserved trials enrolled patients with eGFRs as low as 20 ml/min/1.73 m2, without serious adverse events reported in the lower end of the GFR spectrum. Additionally, the DAPA-CKD trial enrolled patients with chronic kidney disease (CKD), with and without type 2 diabetes (GFR ≥25 ml/min/1.73 m2). Of note, dapagliflozin reduced the risk of kidney failure and cardiovascular death/HF hospitalisation and prolonged survival in CKD patients with or without type 2 diabetes, independently of a history of HF.36 Looking at all these data, it seems reasonable to allow patients with eGFR as low as 20–25 ml/min/1.73 m2 to safely receive these drugs.
Euglycemic Ketoacidosis
The augmented glycosuria after SGLT2I causes reduced glycaemia and reduced production of insulin, with a reduced insulin/glucagon ratio and mild ketogenesis. In cases of seriously impaired insulin production or prolonged fasting, this mechanism may be enough to cause diabetic ketoacidosis (DKA). This euglycaemic DKA shows anion gap metabolic acidosis and ketonuria but without the hallmark sign of hyperglycaemia, which is kept artificially low by maintained glycosuria.37 Euglycemic DKA can be treated with an insulin IV infusion and maintenance of normal blood glucose with glucose infusion, until normalisation of arterial blood gas and ketonuria. This rare and potentially life-threatening adverse event is an absolute contraindication to reinitiating SGLT2I.
Genital Infection
SGLT2I increase the risk of genital infection – mainly Candida fungal infection – already elevated in patients with diabetes, compared to placebo. The infection is preventable with better hygiene and responds well to usual antifungal therapy. Discontinuation of the drug after uncomplicated fungal genital infection does not lead to a better prognosis.38 However, an increased incidence of complicated genital infection (Fournier’s gangrene), a rare and life-threatening perineal bacterial fasciitis, has been demonstrated in 55 patients on SGLT2I identified by the FDA in the US from 2013 to 2019.39 The risk factors are uncontrolled diabetes, obesity, male sex, immunosuppression, poor hygiene and substance abuse. The case reports suggest an augmented risk with SGLT2I, but are too few to generate a specific indication or contraindication. A high index of suspicion is recommended if a patient on an SGLT2I develops genital pain or oedema, which is rare in uncomplicated fungal infection, or unexplained fever.
Lower Limb Acute Ischaemia
A twofold risk of below-the-knee amputation was observed with canagliflozin in the CANVAS trial and in a meta-analysis comprising patients mainly on canagliflozin.40 These results were not observed in other SGLT2I trials, despite sufficient events, so the effect of canagliflozin is not generalisable to other SGLT2I.
Conclusion
Since HFpEF has been recognised, its therapy has been termed as the greatest unmet need in cardiology. From this perspective, the recent FDA approval of sacubitril/valsartan for use in a subset of HFpEF patients, those with ‘below normal’ LVEF, might be viewed as a great achievement, an initial recognition that there is an effective therapy for a subset of HFpEF patients. However, the true revolution in this field is now represented by the EMPEROR-Preserved trial results which show that HFpEF can be successfully treated with the SGLT2I empagliflozin. Importantly, according to a side-by-side examination of the pattern of effects of sacubitril/valsartan and empagliflozin in the PARAGON-HF and EMPEROR-Preserved trials, the magnitude of the reduction in the risk of serious HF outcomes appears to be greater with the SGLT2I compound than with sacubitril/valsartan for most patients with HFpEF.41 Additionally, the beneficial cardiovascular effects of empagliflozin, mainly represented by the reduction in HHF, its safety profile, together with the ease of deployment and use of the drug, will likely facilitate its uptake in clinical practice. The confirmation of the EMPEROR-Preserved trial results from a second Phase III trial in HFpEF and HFmrEF, the DELIVER trial (NCT03619213), testing dapagliflozin versus placebo, are eagerly awaited.
Clinical Perspective
- The SGLT2I empagliflozin represents the first effective treatment for patients with heart failure and mildly reduced ejection fraction, as well as preserved ejection fraction.
- The benefit of empagliflozin in patients with heart failure with midrange or preserved ejection fraction is mainly related to the reduction of hospitalisation for heart failure events, while the impact on cardiovascular disease is not significant.
- The use of empagliflozin has been shown to be safe.
- Confirmation of the EMPEROR-Preserved trial results with the DELIVER trial are eagerly awaited.