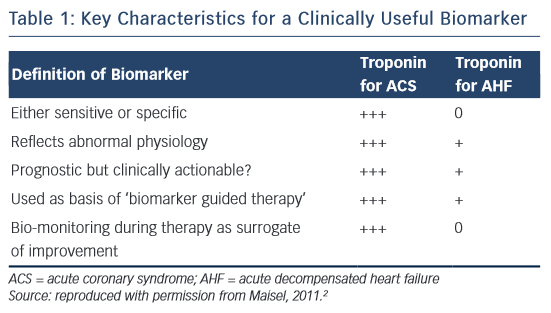
Cardiac troponin (cTn) is the core biomarker for the diagnosis of a myocardial infarct (MI).1 Indeed, as stated in Table 1, it meets all the definitions of a biomarker for acute coronary syndrome (ACS), as suggested by Maisel.2 In the setting of ACS, in addition to its diagnostic use, it is prognostic, clinically actionable and can be monitored during therapy as a surrogate of improvement. In acute decompensated heart failure (AHF), cTn is a valuable biomarker for prognosis; however, it does not aid in the diagnosis of AHF and its role as an actionable biomarker is unclear. Guidelines recommend measuring cTn levels in patients presenting with AHF to evaluate for an ACS event as well as for assessment of prognosis and disease severity.3 There are many reasons that cTn levels can be elevated in heart failure, as well as other disease processes, other than acute ischaemia.1 The implications of troponin in heart failure has been reviewed previously.4,5 This review will focus on the literature on cTn elevation in patients presenting with AHF, with special attention to new high-sense troponin (hsTn) assays.
Physiology of the Troponin Complex and Characterisation of Clinical Assays
The troponin complex plays a critical role in regulating myocyte contraction.6 This complex consists of three subunits that regulate actin–myosin interaction: C, T and I. Troponin T binds the complex to tropomyosin, which is wrapped around actin. Troponin I inhibits the actin–myosin interaction. Troponin C binds calcium thereby regulating actin–myosin inhibition. In the presence of an action potential, intracellular calcium concentration increases and calcium binds troponin C leading to a conformational change in troponin I and tropomyosin, thus removing the inhibition of the actin–myosin interaction. This process occurs in skeletal and cardiac muscle, but not in smooth muscle.
Troponin C is expressed as one isoform that is the same in skeletal and cardiac muscle. The isoforms for troponin T and I are different between skeletal and cardiac muscle; this difference is exploited in clinical assays. One notable aspect of cTn physiology is that not all cardiac troponin T or I (cTnT or cTnI) is bound to the contractile apparatus. Less than 10 % is free in the cytosol, with cTnI consisting of a larger proportion than cTnT.7 This cytosolic pool may play an important role in cTn release in heart failure.
As the isoforms of troponin T and I are specific to cardiac muscle, this has allowed the development of clinical assays for the quantitative assessment of cTnT and cTnI. While several companies make cTnI assays, the cTnT assay is strictly owned by Roche Diagnostics (Indianapolis, IN, USA). Most studies evaluating troponin in heart failure involved the use of early conventional assays. These assays are able to detect troponin at microgram levels. However, assays for both these subunits have gone through multiple generations, with each generation increasing the sensitivity to detect cTn at lower levels. This has led to the latest generation of hsTn assays, with the ability to detect troponin at nanogram and picogram levels. These assays by definition should be able to detect cTn in >50 % of healthy subjects and ideally >95 %.8 Both conventional and hsTn assays have demonstrated important prognostic value in AHF.
Pathophysiology of Troponin in Heart Failure
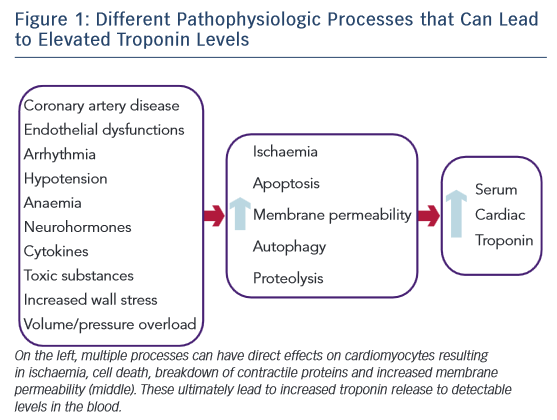
The exact mechanism for increased cTn levels in acute and chronic heart failure is unknown, but many mechanisms are believed to play a role (Figure 1).9 ACS is always a consideration and a Type I MI should be evaluated for in a patient presenting with AHF.3,9 Another possibility is a Type II MI from supply–demand mismatch. This can occur in patients both with and without ischaemic cardiomyopathy. While the primary mechanism is believed to be subendocardial ischaemia, other potential drivers could contribute, including elevated filling pressures, increased wall stress, endothelial dysfunction, tachycardia, arrhythmias, anaemia, and hypotension.5,9 Increased cardiomyocyte stretch and plasma membrane permeability can lead to release of cTn from the cytosolic pool.10,11 In addition, increased wall stress can lead to cardiomyocyte apoptosis, autophagy, and breakdown of the contractile apparatus releasing cTn.12,13 Inflammatory cytokines and neurohormones may be directly toxic to cardiomyocytes as proposed in stress-induced cardiomyopathy.14 Toxic substances such as alcohol, cocaine, methamphetamine or chemotherapy may lead to cTn release by various mechanisms. The exact mechanisms leading to cTn elevation in AHF are unknown, but multiple process including both ischaemic and non-ischaemic mechanisms may contribute.
Troponin and Acute Heart Failure
While troponin level elevation is clearly not diagnostic of acute heart failure, there is overwhelming evidence demonstrating increased morbidity and mortality rates in patients presenting with AHF and an elevated cTn level. Studies have varied in the type of troponin assay used (I or T) as well as the cut-off values used to define a positive test. In addition, as discussed above, as cTn assays have been further refined, they are able to detect cTn at smaller quantities in the blood. Through all of these iterations of troponin assays, cTn has retained its prognostic ability.
Prognostic Value of Troponin with Conventional Assays
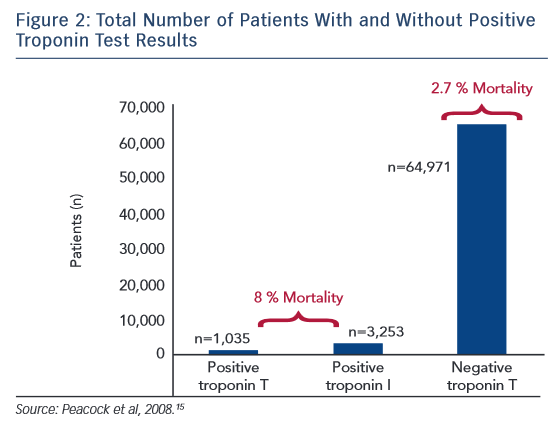
The largest study examining cTn in AHF is the Acute Decompensated Heart Failure National Registry (ADHERE), an observational registry of heart failure hospitalisations at 274 hospitals.15 In an analysis of 67,924 patients, 4,240 (6.2 %) patients had a positive cTn test result on admission (cTnT >0.1 ug/l and cTnI >1 ug/l). Patients with a positive cTn test result had a lower systolic blood pressure on admission and a lower ejection fraction. These patients also had higher in-hospital mortality rates (8.0 %) versus those with a negative result (2.7 %), with an adjusted OR of 2.55 for risk of death (Figure 2). This increased risk persisted when adjusting for different in-hospital therapies, other known risk factors for increased mortality rates, and regardless of whether heart failure was ischaemic or non-ischaemic in origin. A limitation of this study was that the authors could not determine if the cTn level elevation was related to an ACS event. However, this is the largest study examining cTn in AHF showing definitively higher in-hospital mortality rates with an elevated cTn level.
Studies examining only cTnI in AHF have found increased risk of adverse events with an elevated level. In the EFFECT (Enhanced Feedback for Effective Cardiac Treatment) study involving 2,025 patients, a peak cTnI >0.5 ug/l measured in the first 48 hours of hospitalisation was an independent predictor of all-cause mortality at 1 year with an HR of 1.49.16 HR increased with cTnI level (1.1 per 1 ug/l increase) and this risk did not vary between patients presenting with or without acute ischaemia. Smaller studies have also demonstrated increased long-term mortality rates with an elevated cTnI level.17,18 An elevated cTnI level on admission has been associated with lower ejection fraction, higher systolic pulmonary artery pressure, and increased length of hospital stay.17,18 A persistently elevated cTnI level on repeated admissions, especially if >0.04 ng/ml, is associated with increased long-term mortality rates.19
Similar to cTnI, elevated levels of cTnT in patients with AHF have demonstrated increased rates of adverse events. An elevated cTnT level (≥0.1 ng/ml) on admission has been associated with increased risks of heart failure readmission and mortality.20,21 These associations have been observed up to 3 years from the index hospitalisation when cTnT levels first became elevated.22 An elevated cTnT level at the time of discharge from a hospitalisation for AHF predicts an increased risk of heart failure exacerbation, cardiac death, and total mortality (HR of 5).23 Similar to cTnI, cTnT has prognostic use for mortality and morbidity risks.
Serial measurement of cTn levels during a hospitalisation for AHF has been repeatedly shown to have prognostic use. In the PROTECT (Placebo-controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) study, serial cTnT were measured and patients were stratified based on baseline troponin level status (positive: >0.03 ng/ml; detectable: >0.01 ng/ml; negative: ≤0.01 ng/ml) as well as considered to have a conversion if cTn went from negative to detectable on serial sampling.24 On multi-variable analysis, patients with a positive cTn test result or conversion to a positive cTn had an increased risk of cardiovascular/renal rehospitalisation or death at 60 days compared with patients with a negative value. Similarly, in another study, an initially and persistently positive cTn value or a conversion to a positive cTn value was associated with increased risk of mortality, hospital readmission, and the combined endpoint of death and rehospitalisation.25 In the PRESERVD-HF (Pilot Randomized Study of Nesiritide Versus Dobutamine in Heart Failure) study, a significant proportion of patients were found to have an elevated cTn level on admission that rose during hospitalisation before falling or plateauing.26 The initial detectable and peak cTn levels were associated with worse short-term outcomes of death or worsening heart failure. These studies suggest serial measurements of cTn levels during hospitalisation may enhance its predictive value.
Other unique correlations have been found in patients with an elevated cTn level and presenting with AHF. An elevated cTnT level >0.2 ng/ml was associated with a longer left ventricular end-diastolic diameter and end-systolic diameter and a lower ejection fraction.27 An elevated systolic blood pressure and normal cTnI level (<2.0 ng/ml) was effective in identifying a population at low risk of experiencing adverse events that could be monitored in an observation unit.28 As detailed above, an elevated cTn level has been shown to predict mortality and readmissions and this predictive value has been retained as a significant variable in multiple models predicting death and readmission.29,30 Patients presenting with acute cardiogenic pulmonary oedema without MI who have a cTnT level >0.1 ng/ml are associated with a higher long-term mortality risk.31 Overall, the evidence overwhelmingly demonstrates that an elevated cTn level in patients with AHF predicts increased mortality as well as increased risk of readmission and correlation with more severe cardiac dysfunction.
Most studies examining elevated cTn with AHF have focused on patients with heart failure and reduced ejection fraction or combined patients with heart failure with reduced ejection fraction and preserved ejection fraction. Studies focusing solely on patients with AHF with preserved ejection and an elevated cTn level have also shown an association with worse outcomes compared with patients who have an undetectable cTn. In a subgroup analysis of patients with ejection fraction ≥45 % in the ALARM-HF (Acute Heart Failure Global Survey of Standard Treatment) study, an elevated cTnT level (>0.01 ng/ml) was predictive of increase in-hospital mortality rates.32 In another study involving a cohort of patients with an ejection fraction ≥40 %, a cTnT level ≥0.02 ng/ml was associated with worse clinical outcomes of death and rehospitalisation at 6 months with a HR of 1.8 compared with patients whose cTnT was <0.02.33 A study of patients with preserved ejection fraction showed an elevated cTn level to correlate with left ventricular end-diastolic dimension and tissue Doppler Ea wave peak velocity.34 Overall, these studies clearly show that an elevated cTn level in patients with AHF portends poor short- and longterm outcomes regardless of heart failure characteristics.
Prognostic Value of hsTn
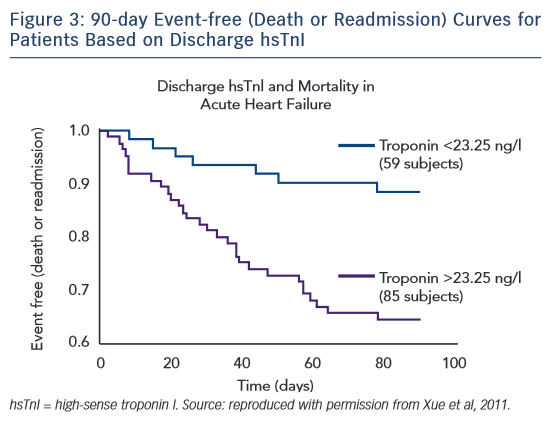
With the development of hsTn assays and the detection of cTn at extremely low levels, a detectable troponin level has continued to show prognostic value in patients with AHF. Yue et al were the first to study serial hsTn I (hsTnI) measurements in patients admitted with AHF, using the highly sensitive VerisensTM RUO Human cTnI assay (Nanosphere). In this study of 144 serial patients presenting with AHF, hsTnI was assessed from admission to discharge with follow-up at 90 days for heart failure-related readmission and mortality.35 Nearly every patient had a troponin level detected above the limit of detection of the assay. Patients with increasing hsTnI levels during treatment had a higher mortality rate than those with a stable or decreasing hsTnI level. Patients with a discharge hsTnI level >23.25 ng/l had an increased risk of readmission and mortality (Figure 3). In an analysis of the ASCENDHF trial, an hsTnI level >0.034 ng/ml on admission was associated with an increased risk of in-hospital mortality, worsening heart failure during admission, and increased length of hospital stay in the first 7 days, but not long-term outcomes at 30 or 180 days.36 A subset of patients had hsTnI measurements taken on admission and 48–72 hours later. Patients with a 20 % increase in hsTnI level had increased risk of 30-day mortality and in a logistic regression model the hsTnI value at 48–72 hours was associated with 30-day mortality, but not the value at admission.
Assays using hsTn T (hsTnT) also have prognostic value similar to that of hsTnI. In a study of 202 patients with AHF, conventional cTnT and hsTnT assays were performed and patients were followed-up for >1 year for mortality.37 Both cTnT and hsTnT had predictive value for death. In patients with a cTnT level <0.03 ng/ml, the addition of a positive hsTnT value, especially if greater than 20 pg/ml, improved risk prediction of death over clinical factors alone. In another study, 113 patients with AHF and a conventional cTnT test result below the cutoff of 0.03 ng/ml on admission were enrolled.38 An elevated admission hsTnT level, especially if >77 pg/ml, was found to be a significant predictor of death, and retained this significance in multivariate analysis with HR of 1.003. In a retrospective analysis of 100 patients admitted with AHF and hsTnT values measured on days 1 and 3, patients in whom the hsTnT levels decreased were found to respond to therapy and return to a compensated state, while those whose hsTnT levels did not change remained decompensated.39 In addition, patients in whom hsTnT levels increased during this time had longer durations of hospitalisation. As hsTn assays become more widely used, these studies demonstrate that in patients with AHF hsTn retains prognostic use similar to conventional cTn assays.
Multimarker Models
Cardiac troponin alone demonstrates significant prognostic use in patients with AHF, but it has also considerable use with other biomarkers in predicting outcomes. Repeatedly, cTn has been paired with B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) and shown prognostic value. In the ADHERE registry, risk of in-hospital mortality was evaluated in patients stratified aacording to BNP level (above or below the median of 840 pg/ml) and cTn level (increased or not). Patients with a BNP level above the median and an increased cTn were found to have an increased risk of in-hospital mortality with an OR of 2.09.40 When stratified by BNP and cTn, an escalating risk was found with: BNP ≥840 pg/ml/cTn not increased (OR, 1.56), BNP <840 pg/ml/cTn increased (OR, 1.69), and BNP ≥840 pg/ml/cTn increased (OR, 3.00). In a smaller study, an elevated NT-proBNP and cTnT were associated with increased rate of adverse events including mortality and rehospitalisation.41 Studies on the optimal time for measuring these biomarkers for predicting long-term outcomes have had conflicting results. One study showed that the measurement of NT-proBNP and cTn levels on admission are stronger predictors of longterm outcomes of death and rehospitalisation than values measured on discharge.42 Yet, another study found the NT-proBNP levels at discharge with a detectable cTnT value and New York Heart Association functional class at discharge were strongest prognostic factors for death and death or cardiovascular hospitalisation.43 Regardless, these studies show the combined prognostic use of cTn with BNP or NT-proBNP for predicting morbidity and mortality.
The addition of C-reactive protein (CRP) to cTn and BNP enhances prognostic ability in multimarker panels. In a study of 102 patients with AHF, the combination of CRP, BNP and cTn had the highest predictive value for mortality at 31 days.44 In a study of 593 patients, the addition of CRP, BNP and cTn to a model using clinical variables significantly improved prediction of 1-year mortality.45 However, only the addition of two of the three biomarkers was needed to improve predictive ability of the model, and the addition of all three biomarkers did not add significant benefit over adding two. HsTn also adds prognostic value in multimarker models. In a study of 107 patients with AHF, soluble ST2, hsTn, and NT-proBNP levels were measured on admission and assessed for predicting risk of death.46 The mortality rate was 0 % in patients with all three biomarkers below their optimal cut-off values and 53 % in patients with elevated levels of all three biomarkers. For each elevated biomarker, the risk of death increased with a HR of 2.64. These studies highlight the independent prognostic value cTn retains in predicting outcomes in multimarker prediction models.
Clinical Relevance
There is no question that an elevated troponin level is prognostic in AHF, and that the higher the level, the worse the prognosis. It is important to remember that cTn is a marker of myocardial necrosis and primarily used to diagnose ACS, which could be the cause of an AHF event. In this role, the percent change in the troponin value on serial measurements and the absolute peak value provide important diagnostic and prognostic information.47,48 Not only is it critical to measure cTn levels to assess for ACS, but the value also provides significant prognostic information for mortality as well as readmission risks and has correlated with other various endpoints. Although finding an elevated cTn helps the clinician risk stratify a patient for outcomes, it does not necessarily help guide immediate therapy. Unanswered questions are depicted in Box 1.
Outside of an ACS event, which can be treated with revascularisation, it is unknown what measures, if any, can be taken to lower an elevated cTn level and decrease the risks of mortality and readmission. Thus an elevated cTn level is limited in its ability to create an actionable trigger for therapy such as increasing neurohormonal blockade, adding antianginals such as nitrates or ranolazine, or increasing diuresis. However, in its ability to help predict prognosis alone and in models, it can help the clinician inform the patient of prognosis as well as consider whether referral to an advanced heart failure specialist/centre for advanced therapies is warranted. In conclusion, overwhelming evidence supports cardiac troponin as a fundamental biomarker in the evaluation of a patient with AHF to assess for ACS and to prognosticate for mortality and readmission, with the latter potentially helping the clinician planning long-term management of the patient.