Acute myocarditis is a sudden inflammation of the myocardium that occurs secondary to exposure to toxic substances, medications, infections, systemic diseases and profound immune system activation, among others (Table 1).1-3 Globally, it affects 4–14 people per 100,000 yearly.2 It constitutes a relatively common cause of sudden cardiac death in young people, accounting for 6–10% of autopsy-based case series.3 It also accounts for 0.7 % of cases receiving orthotopic heart transplantation (OHT) and 9–16% of patients with non-ischaemic cardiomyopathy.1,4 Overall, mortality due to acute myocarditis is estimated at 1–7%.2 Acute myocarditis can be categorised according to its clinical presentation, histopathology or specific aetiology or causative agent (Table 1).2,3 The exact mechanism determining the transition from the initial trigger to myocardial inflammation, and from acute myocardial injury to chronic dysfunction, remains unclear. There is currently an unmet need to diagnose patients with myocarditis accurately and early, and to identify individuals at the highest risk of adverse cardiovascular (CV) events and those who might need further advanced heart failure (AHF) therapies. In this review, we elaborate on the classification of acute myocarditis based on its clinical presentation and histopathology. This is supplemented by the presentation of a challenging case of acute myocarditis that illustrates the clinical, biochemical, imaging and histopathological red flags highly suggestive of a more severe clinical course of acute myocarditis.
Case Presentation
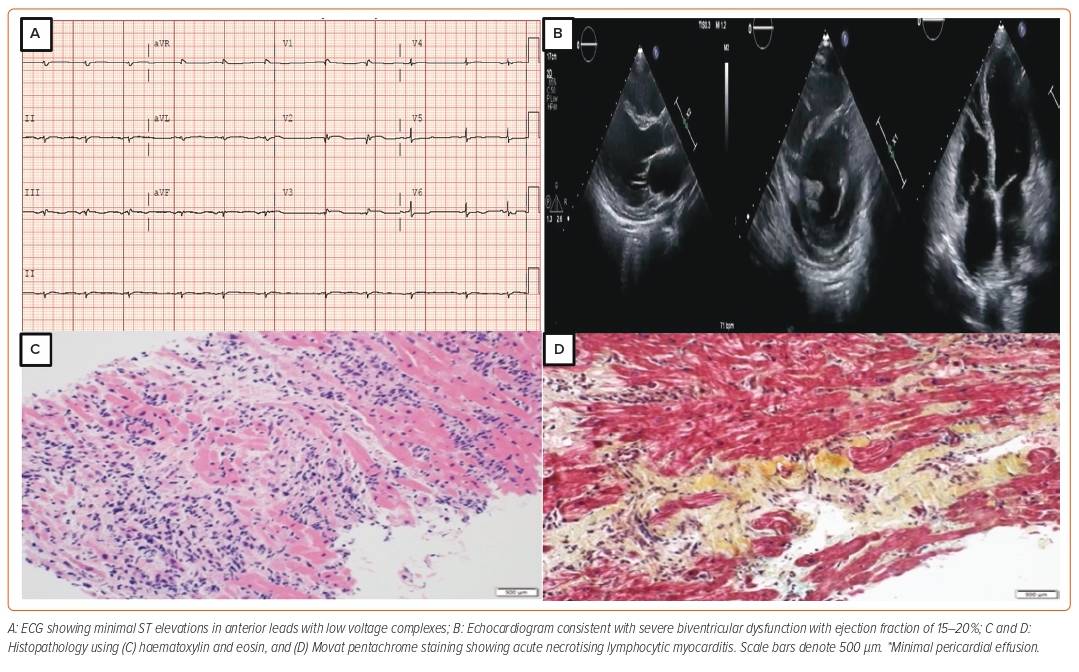
A 38-year-old woman with no significant past medical history presented to the emergency department with a 2-day history of dry cough and worsening shortness of breath associated with dizziness. She had a recent travel history to Saudi Arabia 2 months prior, where she developed disseminated hives from an unknown cause. She was hospitalised and treated with systemic steroids abroad. She had no unwell contacts and no family history of cardiomyopathy. At presentation to our emergency department, she was in frank pulmonary oedema. Laboratory tests revealed slight rise in troponin T values (first reading 1.8 µg/l; second reading 2 µg/l; reference <0.06 µg/l); the ECG showed minimal ST elevations in the anterior leads, whereas the bedside echocardiogram showed globally reduced left ventricular ejection fraction (LVEF) of 15% and moderately reduced right ventricular (RV) systolic function (Figure 1). Subsequent coronary angiogram showed normal coronaries. Her pregnancy test was negative and her eosinophils in blood counts were within the normal range. Immunoglobulin E levels were also normal.
Cardiac magnetic resonance (CMR) showed extensive myocardial oedema with several small foci of fibrosis in the interventricular septum and posterolateral wall but without hyperaemia. She was treated as a case of acute viral myocarditis. Over her treatment course, she rapidly deteriorated and progressed to cardiogenic shock (CS; Society for Cardiovascular Angiography and Intervention [SCAI] stage C, Interagency Registry for Mechanically Assisted Circulatory Support III), supported with intravenous dobutamine and norepinephrine. She also developed ventricular tachycardia (VT), requiring pharmacological cardioversion with IV amiodarone and mechanical ventilation because of her worsening respiratory status.
In light of this worsening clinical condition, she was started on IV pulse dose steroids. A pulmonary artery catheter confirmed the clinical findings of severe biventricular failure with elevated filling pressures and a low cardiac output state. After initial stabilisation, her condition deteriorated, requiring veno-arterial extracorporeal membrane oxygenation (VA-ECMO) support and an intra-aortic balloon pump (IABP) to vent the left ventricle (LV). She was listed as status 2 for AHF therapies (LV assist device/OHT). Over the course of the next 2 weeks, VA-ECMO was successfully weaned. Repeat echo showed severe biventricular failure with LVEF of 10% and severely reduced RV function with no signs of recovery. Ultimately, she underwent a successful OHT 6 weeks after her initial presentation. A biopsy of her explanted heart showed inflammatory infiltrates consisting predominantly of lymphocytes, consistent with acute necrotising lymphocytic myocarditis. At a 3-year follow-up visit, she was doing well with no signs of rejection on subsequent routine biopsies. On coronary angiogram, no evidence of coronary artery vasculopathy was noted and the patient was adherent to her immunosuppressive regimen.
Clinical Presentation: Fulminant versus Non-fulminant Acute Myocarditis
Acute myocarditis may present with heterogeneous signs and symptoms varying from mild influenza-like illness to acute decompensated heart failure and CS.3 Approximately 82–95% of adult patients present with chest pain, 19–49% with dyspnoea and 5–7% with syncope.2 Variability in clinical presentation depends on the degree of inflammation and scarring, ultimately leading to dilated cardiomyopathy.3 Worse New York Heart Association class and syncope are associated with poor outcomes.5
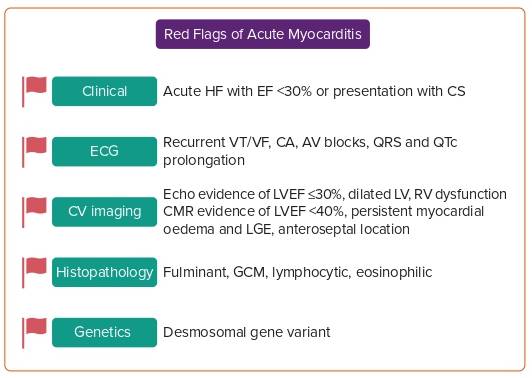
Acute (<1-month duration) clinical presentation for patients presenting with a severe form of myocarditis includes rapidly progressive decompensated heart failure or CS, severely depressed LVEF to <30%, haemodynamic or electrical instability (in the form of VT, ventricular fibrillation or complete heart block). Fulminant myocarditis (FM) is a form of acute, rapidly progressive myocarditis (<2 weeks duration) characterised by severe LV systolic dysfunction requiring inotropes and/or mechanical circulatory support (MCS). A study by Ammirati et al. showed that, among 220 patients with histologically proven acute myocarditis with LV dysfunction, those with FM had higher rates of cardiac death and requirement for OHT compared with the non-FM variety (28% versus 1.8%; p=0.0001) and at 7-year follow up (48% versus 10%; p<0.0001).6 Among those presenting with FM, giant cell myocarditis (GCM) had a significantly worse prognosis than eosinophilic and lymphocytic myocarditis. Hence, it is prudent that endomyocardial biopsy (EMB) should be performed early in the disease course and appropriate treatment started. Patients should be triaged and early referrals should be made to centres capable of providing advanced MCS. Among patients with fulminant presentation, those who require OHT are relatively younger (<45 years), sicker and more often require biventricular MCS.4 Survival is similar, while chances of recovery are double those for other forms of heart failure among patients listed for OHT, the exception being GCM.7
Ammirati et al. reported in their multi-centre registry that, among 443 patients presenting with acute myocarditis, 26% presented with either progressive LV systolic dysfunction, ventricular arrhythmias (VA) or CS representing a more fulminant nature.8 The remainder of the patients had less severe forms and, therefore, were less likely to require OHT and had lower rates of complications and death at 5-year follow-up.8 Figure 2 summarises key red-flag signs of acute myocarditis.
Biochemical Markers
Among patients with acute myocarditis, a weak correlation exists between the degree of troponin leak and the severity of cardiac dysfunction or persistence of late gadolinium enhancement (LGE).9,10 Markers of inflammation, such as C-reactive protein, are positive in 80–95%.8 Persistently elevated erythrocyte sedimentation rate may suggest autoimmune involvement, whereas eosinophils in the bloodstream may hint toward eosinophilic myocarditis.11 In a study by Berg et al. among patients presenting with acute myocarditis, there was no correlation between improved LGE on CMR and decreasing levels of cardiac enzymes and inflammatory parameters at 3 months follow-up.10
Müller et al. defined the association of two proinflammatory proteins, S100A8 and S100A9, with Coxsackie B virus-myocarditis in five patients.12 Elevated protein levels were found in heart tissue when acutely ill, while levels decreased significantly as LV function improved.12 Further research may assist in determining whether these markers hold true value among those presenting with other causes of viral myocarditis.
Electrocardiographic Findings
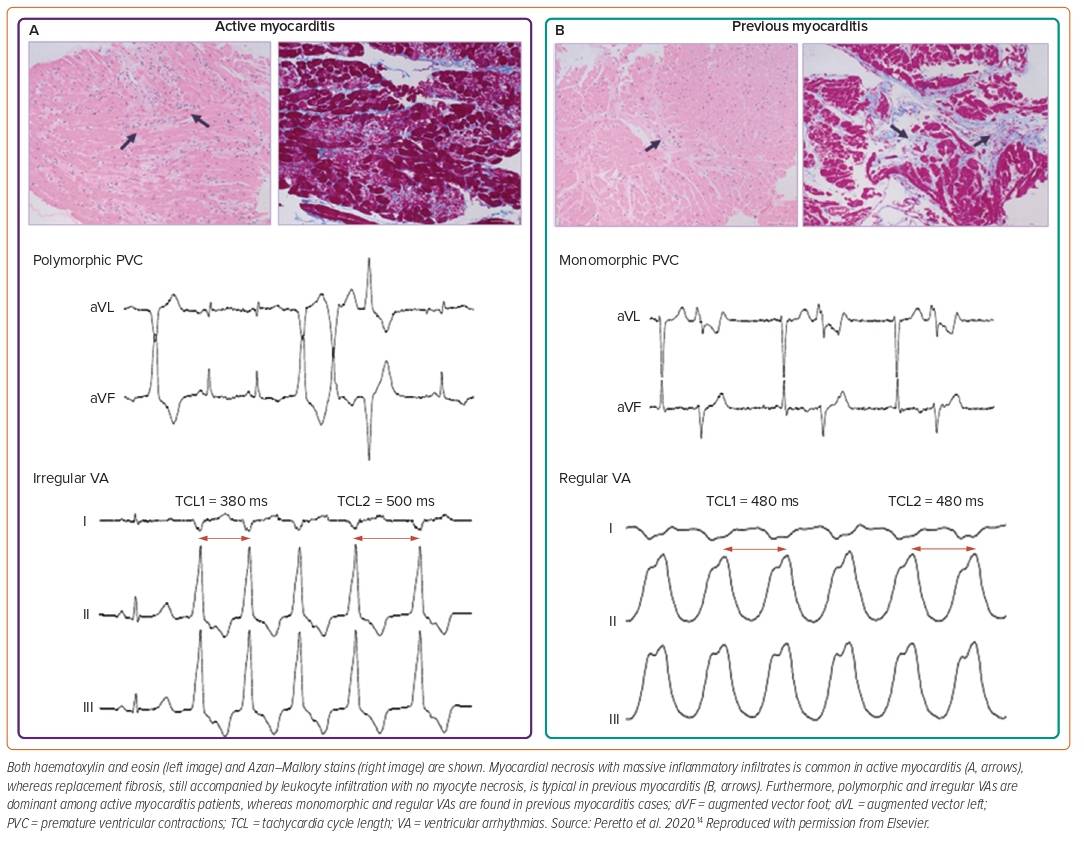
ECG is widely used as a screening tool despite its low sensitivity.1,13 Findings may vary from nonspecific T-wave inversions to ST-segment elevations mimicking an acute MI (concave ST elevations rather than convex in myocardial ischaemia and diffuse without reciprocal changes).1 Potential red-flag findings include QTc prolongation of >440 ms, an abnormal QRS axis, ventricular ectopic beats and prolonged QRS duration of >120 ms have been found to be independent predictors for cardiac death or OHT.5 Similarly, atrioventricular (AV) block, symptomatic bradycardia or tachycardia and VA may suggest high-risk forms (giant cell variant, Lyme- and sarcoid-related myocarditis). Furthermore, polymorphic and irregular VA are dominant in acute myocarditis, while monomorphic and regular VA are more common in chronic myocarditis (Figure 3).6,14
Ablation for drug-refractory VT should be avoided during the acute phase of myocarditis. In a study by Peretto et al. of 125 patients with myocarditis and drug-refractory VT, those who underwent ablation in the active phase (<3 months) had higher rates of VT recurrence at 1-year follow-up compared with those who underwent ablation with previous history of myocarditis (i.e., >12 months prior).15
Imaging Findings
Echocardiogram
Echocardiographic findings such as impaired LVEF on admission, RV dysfunction, the presence of pericardial effusion, increased wall thickness, mild segmental hypokinesis, especially involving inferior and inferolateral walls, abnormal diastolic function, abnormal tissue Doppler imaging and abnormal myocardial echogenicity may suggest active myocarditis. Among these findings, LVEF on admission is the strongest prognostic marker to determine the outcome of patients admitted with acute myocarditis.6,8 Those presenting with either preserved or mildly reduced ejection fraction generally have a good prognosis and follow a benign clinical course.8
Cardiac Magnetic Resonance
CMR has emerged as an excellent and efficient non-invasive diagnostic tool that aids in tissue characterisation (quantification of inflammation, fibrosis), which helps to differentiate between active versus inflammatory myocarditis.16 To reliably rule in or rule out myocardial inflammation, CMR should be performed within 2–3 weeks from the onset of symptoms, as accuracy is lower during the initial few days.3 Similarly, it should not be performed after 4 weeks, as oedema tends to settle after that period.17
Lake Louise criteria published in 2009 incorporated three findings of myocardial inflammation:1) hyperaemia-intense signal in early gadolinium enhancement images; 2) tissue oedema, that is, increased myocardial T2 relaxation time or an increased signal intensity in T2-weighted images; 3) and fibrosis based on LGE images.18 If two of these three criteria are positive, acute myocarditis can be diagnosed with 74% sensitivity and 86% specificity.18 Updated Lake Louise criteria include T2 mapping for oedema and native T1, as well as extracellular volume for inflammatory injury. This was validated in a study that confirmed enhanced sensitivity to 87.5% and a high specificity of 96.2% to accurately diagnose acute myocarditis.19 Additional findings of increased T2 relaxation time may suggest greater myocardial water content and a greater likelihood of improved cardiac function.20
Gräni et al. prognosticated CMR among 670 patients presenting with suspected myocarditis to estimate cardiac event-free survival.21 The presence of LGE was associated with more than twice the risk of major adverse cardiac events (MACE), which was a combination of death, hospitalisation from heart failure and recurrent VAs. Septal and mid-wall LGE showed the greatest associations with MACE, while a patchier distribution was associated with a three-fold increased risk for MACE. Similarly, the extent of LGE (i.e. >10%) was associated with a 79% increased risk for MACE, whereas a normal CMR study was associated with low annual rates of MACE and cardiac death (0.8% and 0.3%, respectively).
The presence and location of LGE in the mid-layer of the septum and low LVEF at baseline are the strongest negative predictors of outcomes.21,22 CMR is also useful in the follow-up of patients with acute myocarditis and is generally performed 6 to 12 months after the index event.3,14 The disappearance of oedema is frequent at follow-up (up to 84% of cases), whereas LGE generally persists (in up to 89%), which may reflect post-inflammatory fibrosis.14,23 The persistence of LGE and disappearance of oedema are markers of unfavourable prognosis.21,23
18F-Fluorodeoxyglucose PET Scan
Generally, 18F-fluorodeoxyglucose PET (FDG-PET) is not indicated unless the patient cannot undergo CMR (either because of irregular heartbeat or ICD-related artifacts), or in cases of suspected systemic autoimmune disease where other organs could be involved by the inflammatory process or findings that are discordant between EMB and CMR.24 A recent observational prospective study by Peretto et al. showed that, among patients who underwent FDG-PET for arrhythmic myocarditis diagnosed by EMB or CMR, anteroseptal distribution was associated with a greater occurrence of VAs and AV blocks and was more likely to be associated with cardiac sarcoidosis.25 FDG-PET was able to accurately diagnose cardiac sarcoidosis in 100% of cases with predominant anteroseptal location, followed by lymphocytic myocarditis in 96% of cases (predominant inferolateral location) among patients who had EMB performed. Additionally, PET allows myocarditis monitoring to guide immunosuppression withdrawal.
Endomyocardial Biopsy and Histopathology
EMB is an invasive procedure with a complication rate of 1–2% in high-volume centres and 8–9% in low-volume centres.26 EMB is performed in 3.6% of overall cases with myocarditis based on data obtained from the US Nationwide Inpatient Sample database.26 Trends for EMB have declined because of the increasing utility of non-invasive imaging modalities. EMB was associated with increasing length of stay, risk of complications such as cardiac tamponade and worse mortality in a US-based data registry.27 The sensitivity of EMB is low as the sampled tissue may not always correspond to the distribution of inflammation. Immunohistochemistry-specific antibodies for leukocytes (CD45), macrophages (CD68), T cells (CD3) and their main subtypes, helper (CD4) and cytotoxic (CD8) cells, and B cells (CD19/CD20) can increase the sensitivity of EMB.1
A combination of history, highly sensitive troponins and CMR have greatly improved the non-invasive diagnostic accuracy of acute myocarditis, resulting in the identification of more low-risk patients than previously, when the diagnosis was mainly based on EMB, which was performed more often in sicker patients.16
EMB is still fundamental in specific forms of myocarditis, such as in patients presenting with acute severe heart failure or CS, VAs or high-grade AV blocks (fulminant variant); in the presence of peripheral eosinophilia; and in the setting of immune checkpoint inhibitors, where the appropriate diagnosis has implications for patients receiving additional cancer therapy.28,29 Diagnostic yield significantly increases if EMB is performed within 2 weeks of presentation with acute myocarditis and the above findings. In the following sections, we present special aetiologies of acute myocarditis from a histopathological standpoint.
Aetiology and Classification
Viral Myocarditis
Enteroviral (Coxsackie virus) myocarditis is an example of virus-mediated myocarditis, as viral replication can cause direct cardiomyocyte injury. Respiratory viruses, such as influenza and coronaviruses, are examples of common viruses that can trigger immune-mediated lymphocytic myocarditis in the absence of a viral genome in the myocardium.30 Parvovirus B19 has also been implicated in both acute and chronic myocarditis, both in children and adults. Among patients with biopsy-proven viral myocarditis, long-term mortality is 19% at approximately 5 years.22 Parvovirus B19 was the common virus identified (56%), followed by the human herpes-6 virus (24%) among patients with biopsy-proven viral myocarditis. During the COVID-19 pandemic, infection with the virus or its mRNA vaccine has been linked with acute myocarditis, with a lower risk of developing acute myocarditis among vaccinated individuals.2 Limited data are available to conclude whether acute treatment of the infection might affect long-term myocardial recovery and LVEF.2,31,32 Further surveillance and monitoring with CV imaging are certainly warranted in these cases.32
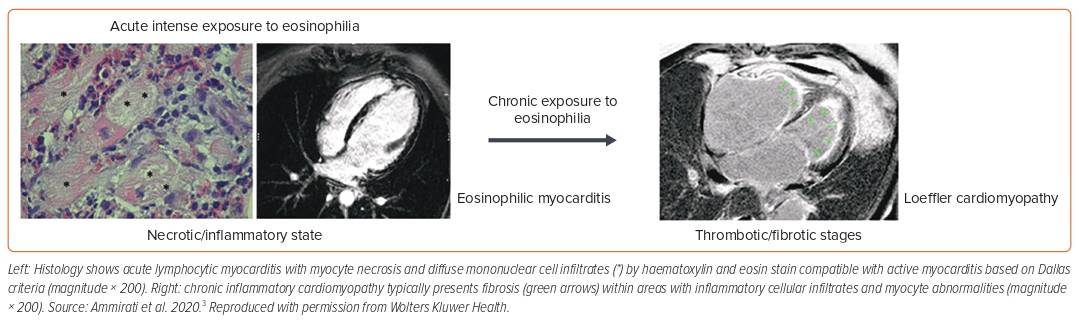
Eosinophilic Myocarditis
Eosinophilic myocarditis (EM) is often unrecognised but is associated with high rates of mortality, requiring OHT (26% at 2 months data) among those with fulminant presentation.6 Causative factors include systemic conditions such as eosinophilic granulomatosis with polyangiitis, hyper-eosinophilic syndrome, parasitic infection (Toxocara canis transmitted by raw meat) or hypersensitivity reactions to chemicals (β-lactam antibiotics, clozapine, carbamazepine).3 Eosinophilia can be evident in the course of the disease but is absent in about 25% of patients at admission.11 The presence of LV thrombus is seen in approximately 28% of cases (Figure 4). The LGE pattern on CMR is subendocardial in 57% of histologically proven eosinophilic acute myocarditis cases.10
Giant Cell Myocarditis
GCM is a form of rapidly progressive myocarditis associated with a high fatality rate (approximately 85% death or requirement for a heart transplant at 3 years).3,6 GCM accounts for 1 in 200 cases of myocarditis and is associated with autoimmune disorders such as inflammatory bowel disease and thyroiditis in approximately 20% of patients.3,7 It is characterised by myocardial destruction mediated by a large number of cytotoxic T cells, macrophages, giant cells and eosinophils. GCM frequently presents as CS and with VT or complete AV block.33 In a single-centre case series of GCM, among patients who underwent CMR, LGE was present in 96% of patients, and it tended to be widespread, involving all layers of the myocardium because of extensive underlying inflammation and/or fibrosis.13,34
EMB is generally the first diagnostic tool and immunosuppressive therapy should be initiated early, which can achieve remission in up to two-thirds of cases.35 Patients are at high risk of recurrent VT, and placement of an ICD is generally recommended in all patients, including those with full recovery of LVEF.36 Among those requiring advanced MCS such as VA-ECMO, two-thirds of patients survive.37 Rates of relapse are high, including in those who acquire remission on immunosuppressive therapy through to those who require OHT.38
Genetics
Red flags for a genetic aetiology include a positive family history of myocarditis, arrhythmogenic RV cardiomyopathy or sudden cardiac death. In such patients, testing for desmosomal gene variants can be helpful.39,40 Ammirati et al., in their recent publication, analysed the series of genetic probands admitted with suspected myocarditis.41 Patients with acute myocarditis who were desmosomal gene variant-positive had higher rates of VAs (specifically non-sustained VT on telemetry monitoring), recurrence of myocarditis (63% at 5 years follow-up) and persistence of LGE on CMR at follow-up visits compared with acute myocarditis patients who were gene variant-negative.41 Among the desmosomal gene variants, most (87%) were variants of the desmoplakin (DSP) gene, while others included the DSG2 gene (6.5%) and PKP2 gene (5%). Another study on myocarditis identified putative deleterious variants in (16.2%) of patients, with the titin gene being the most common variant, followed by the DSP gene.42 Genetic studies should be considered when these strong red-flag signs are observed among patients presenting with acute myocarditis.
Back to Our Case
This review addresses key red-flag clinical, laboratory and imaging findings that can identify high-risk patients with acute myocarditis compared with the benign variant. Key red-flag findings in our case include presentation with CS with rapid worsening to Stage D SCAI shock classification, biventricular failure (LVEF of 15%) with moderate to severely reduced RV function, CMR evidence of extensive myocardial oedema with several areas of fibrosis, histopathological evidence of necrotising lymphocytic myocarditis, VT during in-hospital stay and the need for inotropes and MCS (VA-ECMO and IABP). We believe these findings can greatly aid in identifying fulminant versus non fulminant variant of myocarditis and – with early intervention – can improve outcomes.
Limitations
This review has several limitations. The article is primarily focused on identifying key red-flag signs in clinical assessment, laboratory and imaging findings. Hence, we do not focus on individual types of myocarditis and their specific management. Second, COVID-19-associated myocarditis was discussed superficially.
Conclusion
This review addresses key red-flag features in clinical, laboratory, imaging and histopathological characteristics that can greatly assist in differentiating between fulminant and non-fulminant variants of acute myocarditis. Early identification based on signs can greatly improve outcomes, particularly in fulminant variants of the condition. 











Comments