Dear Editor,
We read with great interest the review article by Alzaabi et al., which discusses multiple biomarkers associated with heart failure (HF), including those related to oxidative stress, the extracellular matrix, renal function, inflammation, cardiac peptides and other novel biomarkers.1 The authors describe the clinical implications of these biomarkers for evaluating the risk of HF development as well as HF severity, prognosis and therapy responsiveness.1 In this letter, we highlight elevated red cell distribution width (RDW) as a prognostic marker in HF and a potential predictor of therapy responsiveness.2 We review the most relevant studies regarding RDW as a prognostic marker in HF, discuss mechanisms responsible for RDW elevation and explore the relationship between high RDW and HF.
RDW measures the heterogeneity of the distribution of red blood cell size. The term ‘width’ is misleading, as the value is not derived from the width of the red blood cells (RBC), but rather from the width of the distribution curve of the corpuscular volume. A high RDW, therefore, implies a large variation in RBC size that is also known as anisocytosis, and a low RDW indicates a more homogeneous population of RBC sizes.2,3 RDW is routinely assessed as part of the complete blood count and is calculated as RDW = (SD/mean corpuscular volume) × 100, with reference values of approximately 11–15%.4
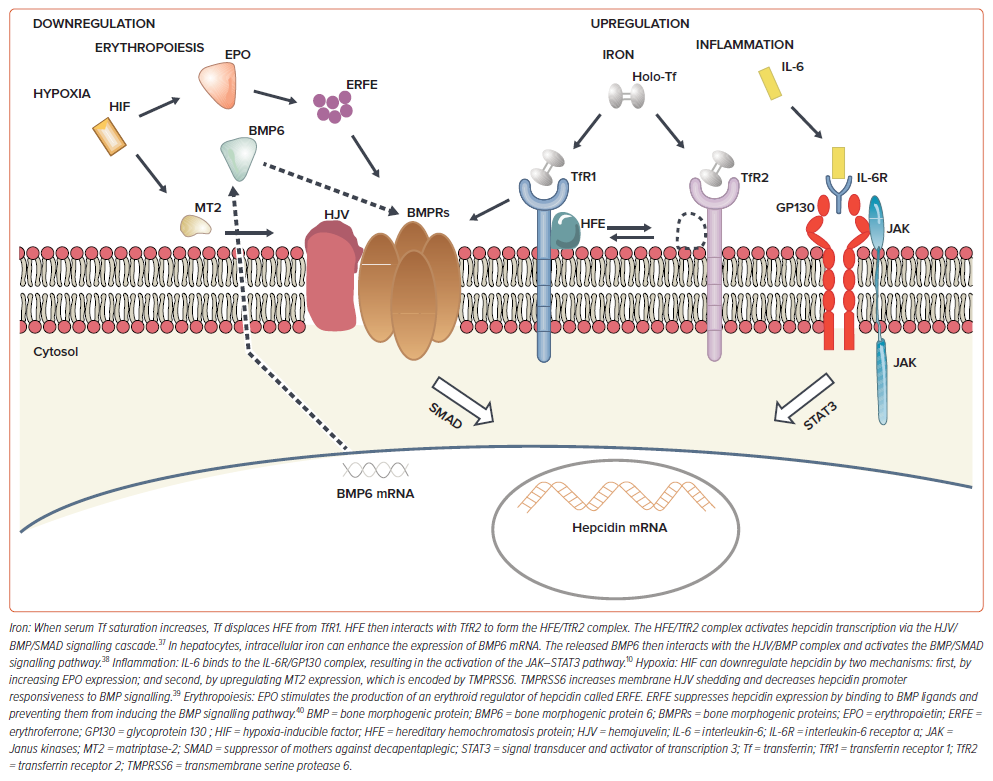
Hepcidin is a 25 amino acid peptide hormone produced by the liver. It is the main regulator of iron homeostasis under various pathological conditions and affects both erythropoiesis and RBC turnover rate.5 Hepcidin binds the iron exporter ferroportin, which is expressed in enterocytes, macrophages, hepatocytes and erythrocytes.6 Hepcidin mRNA expression decreases during hypoxaemia due to decreased ambient oxygen or to anaemia caused by bleeding or haemolysis. Conversely, hepcidin mRNA expression increases in response to inflammation and increasing serum iron levels.5,6 A different variation in the size of the RBC population generates a high RDW.2–4
Hypoxia can lead to the production of hypoxia-inducible factors, which increase erythropoietin (EPO) production depending on the severity of hypoxaemia. EPO production can enhance the self-renewal of colony-forming unit-erythroid by approximately 170-fold, causing the early release of young cells into circulation.7 A cohort study of patients admitted to intensive care with acute respiratory failure found that reticulocyte levels gradually increased as RDW rose.8 Therefore, EPO not only increases the rate of RBC formation but also the volume of RBCs, resulting in a higher RDW.9 Hence, either the downregulation or upregulation of hepcidin can produce a high RDW (Figure 1).
Considerable evidence supports the role of interleukin (IL)-6 in regulating hepcidin expression by directly binding of signal transducer and activator of transcription 3.10 IL-6 is a well-known cytokine that plays an important role in chronic inflammation states, such as infection, cancer, immune-related disorders, diabetes, HF and cardiovascular disease.11–14
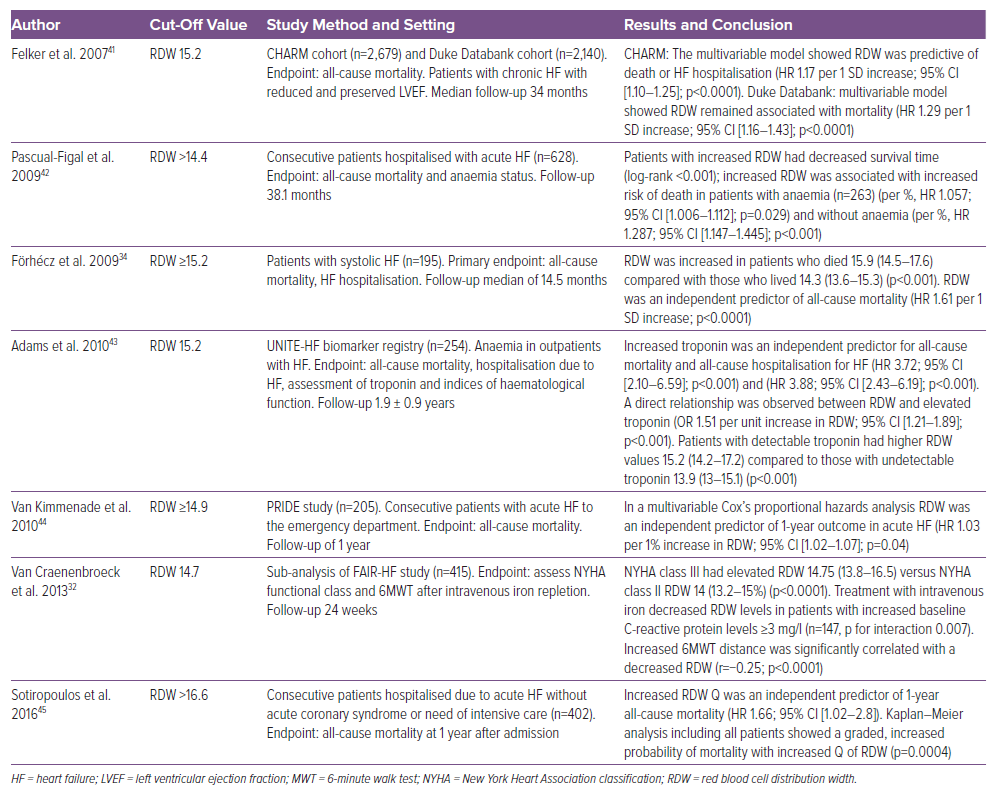
From a practical perspective, a high RDW can be due to ineffective erythropoiesis, inflammation, hypoxaemia and/or impaired iron availability.2,3 Therefore, RDW is not specific to HF since an elevated RDW can be associated with haematologic and autoimmune disorders, oncological disease, critical conditions such as systemic inflammatory response syndrome and acute respiratory distress syndrome, acute bleeding or transfusions.2,3,9,15–18 Numerous studies have shown that an elevated RDW is associated with an increased risk of all-cause mortality and adverse cardiovascular events, such as MI, stroke and hospitalisation due to HF decompensation. Additionally, elevated RDW has been linked to an increased risk of AF, with some studies suggesting it is a potential predictor of AF recurrence after catheter ablation.2,3,19,20 An elevated RDW is, therefore, a prognostic marker in HF (Table 1). Moreover, a cohort study in Massachusetts reported that high RDW is associated with an increased mortality risk for patients with COVID-19; patients whose RDW increased during the first week of admission experienced higher mortality rates than those whose RDW remained stable. Increased mortality was related to hypoxaemia, impaired iron availability, elevated ferritin levels and increased IL-6 due to high systemic inflammation, a common occurrence in acute distress respiratory syndrome with or without COVID-19.21,22 The study demonstrated that RDW increases in both chronic and acute inflammatory states.21
Iron deficiency (ID) is found in 37–50% of patients with stable chronic HF, suggesting dysregulation of iron metabolism in HF.23,24 It is unclear whether these changes are maladaptive and pathologic, or compensatory and protective for the cardiomyocytes. Furthermore, macrophages play an important role in iron homeostasis by storing excess iron in iron overload diseases or promoting iron retention in chronic inflammatory conditions such as HF.10,14 This process is mediated by IL-6, which upregulates the hepcidin–iron axis.10,14,25 High RDW can occur in ID, which can be either absolute (low ferritin) or functional (normal/high ferritin), with or without concurrent anaemia.25,26 Contemporary HF research leans toward transferrin saturation (TSAT) as a better and more appropriate marker of functional ID than ferritin.27
Large-scale clinical trials have demonstrated improvements in New York Heart Association functional classification following iron replacement therapy in patients with HF and iron deficiency.28–30 Accordingly, the European Society of Cardiology guidelines recommend iron replacement therapy in patients with HF and ID, defined as serum ferritin <100 μg/l or ferritin ≥100 μg/l and TSAT <20%.31 Interestingly, a sub-analysis of the FAIR-HF study revealed that high RDW was associated with decreased TSAT and increased C-reactive protein. Treatment with intravenous ferric carboxymaltose in patients with ID and chronic HF reduced RDW. Furthermore, the increase in 6-minute walk test distance after 24 weeks was significantly correlated with a decrease in RDW (r=−0.25, p<0.0001), even after adjusting for changes in haemoglobin.32
From our perspective, the mechanisms of progressive HF may be intertwined with increasing myocardial iron deficiency, which is associated with reduced myocardial oxygen consumption and impaired myocardial metabolism, leading to a worse prognosis. It is, therefore, crucial to identify patients with ID and closely monitor their treatment response.
Notably, some studies have demonstrated that high RDW correlates with increasing IL-6 and impaired iron availability due to iron retention by macrophages.20,33,34 Consistent with this, the BioStat-CHF study reported that elevated IL-6 levels were found in over 50% of patients and were associated with ID.14 Consequently, RDW serves as a highly sensitive marker for ID.35
Progressive renal and hepatic dysfunction, decreased peak oxygen uptake, and high systemic inflammation are commonly seen in advanced HF and can contribute to ineffective erythropoiesis.36
In conclusion, RDW is a biomarker of red blood cell dysfunction, indicating elevated systemic inflammation, hypoxemia and/or ID. ID may be absolute or functional, the latter due to impaired iron mobilisation from stores caused by inflammation-driven factors, including IL-6, hepcidin and macrophage iron retention. Finally, RDW stands out as a valuable marker because it is included in routine full blood counts, making it both low-cost and easy to obtain at the bedside. From a clinical perspective, an elevated RDW implicates a worse HF prognosis due to an increased risk of both all-cause mortality and adverse cardiovascular events. Patients with a high RDW may also have ID and be at risk for developing ineffective erythropoiesis. Moreover, in patients with ID confirmed by serum ferritin <100 μg/l or TSAT <20%, a reduced RDW following iron replacement therapy is associated with clinical improvement. Thus, RDW may also predict iron replacement responsiveness in HF.