Heart failure (HF) is an epidemic with an increasing prevalence and an absolute mortality rate of approximately 50 % within 5 years of diagnosis. Imaging plays a main role in HF diagnosis, assessment of aetiology and treatment guidance. This article reviews current HF applications for all the available non-invasive imaging modalities: echocardiography, cardiovascular magnetic resonance (CMR), nuclear imaging-positron emission tomography (PET) and single-photon emission computed tomography (SPECT) and computed tomography (CT). Echocardiography, with its recent developments, such as 3D echo, is the main imaging test used in the evaluation of HF patients, given its availability and reliability in assessing cardiac structure and function. CMR allows the characterisation of myocardial tissue, in addition to providing information on the structure and cardiac function, so it is a great help in the determination of HF aetiology and may predict patient outcomes. Nuclear imaging can detect ischaemia and viability and can obtain additional prognostic data. Cardiac CT is a reliable method for the detection of coronary artery disease (CAD), and recent advances have in turn provided information about function and myocardial perfusion. In general, available imaging methods yield reliable measures of cardiac performance in HF, and recent advances allow detection of subclinical disease. In the following pages, current indications and possible future applications of each one of the above mentioned modalities are developed with further detail.
Echocardiography
Although echocardiography is a relatively ‘ancient’ technique, its versatility makes it unique in the provision of volumes, function, haemodynamics or valvular regurgitation. In addition, because of its availability, safety and low cost, echocardiography is the first and most widely used test for the diagnosis, selection of appropriate treatment and prognosis of HF.1 Echocardiography is also the most used imaging method in the reassessment of HF patients. It remains unclear when to repeat echocardiographic exams, but it is deemed appropriate at least in patients with worsening symptoms.
Systolic Function
Global left ventricular (LV) function is of paramount importance regarding therapeutic decisions. Visual estimation of ejection fraction (EF), the Teichholz and Simpson methods, have been widely validated.2,3 However, in 2D imaging, because of its operator-dependent nature, repeated testing has a high probability of producing variable volume and EF results.
The apparition of 3D fully sampled matrix transthoracic echocardiography has enabled easier acquisition of images,4 simplifying its routinary application. There have been many studies comparing 2D and 3D echocardiography (2DE and 3DE) and a ‘reference’ standard (generally CMR). A recent meta-analysis of all 3DE studies evaluating LV volumes and EF demonstrated that 3DE generally underestimated volumes, but not as significantly as 2DE. There was also less variability than 2D compared with CMR.5 Nonetheless, 3DE echocardiography may be challenging and not practical in patients with low image quality (e.g. critical patients), in whom 2DE measures are more realiable. 3DE is also a less standardised techique than 2DE, and because of this reason most laboratories use the more universally applicable 2DE measurements in clinical practice. Only recently, large analyses of LV parameters using 3DE in large cohorts of healthy individuals have been published to establish race, age and gender-specific reference ranges to facilitate the standardisation of this technique.6
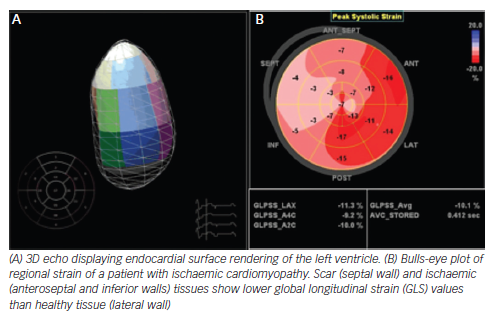
The most commonly used strain-based measure of LV global systolic function is global longitudinal strain (GLS), which is usually assessed by speckle-tracking echocardiography and describes the relative length change of the LV myocardium between end-diastole and end-systole. The preponderance of currently available data is for midwall GLS, and, although there is a wide heterogeneity in the published literature, a peak GLS in the range of 20 % can be expected in a healthy person.7,8 It is essential to know the pitfalls and limitations of this method, specially the critical importance of optimised echocardiographic recordings and avoidance of apical foreshortening (which may significantly change the value of the obtained measurements).
Strain is of special interest in two clinical scenarios. Studies in patients with chemotherapy demonstrate that early alterations of myocardial deformation precede significant change in EF. A 10 % to 15 % early reduction in GLS during therapy is the most useful parameter for the prediction of cardiotoxicity, defined as a drop in LVEF or HF.9 Moreover, 2D speckle-tracking imaging could be useful in differentiating cardiac amyloidosis from other causes of LV hypertrophy by showing reduced basal strain and regional variations in LS from base to apex and a relative ‘apical sparing’ (average apical LS/[average basal LS + mid- LS]) pattern. GLS also provided incremental prognostic value over N-terminal of the prohormone brain natriuretic peptide (NT-proBNP), cardiac troponin and other clinical variables.10,11 In addition, although currently at an experimental level, strain imaging may help to detect subclinical cardiac dysfunction, e.g. in patients with diabetes.
Diastolic Function
Comprehensive diastolic assessment by tissue Doppler imaging, transmitral flow velocities and deceleration time, pulmonary venous Doppler, left atrial (LA) size and pulmonary artery pressures is mandatory in the evaluation of suspected HF. Nonetheless, the concordance between observers in this setting is limited.12
LA volume has a stronger association with outcomes compared with anteroposterior diameter. 3DE is more accurate than 2D assessment and provides fewer underestimated values in connection with CMR.13
In some patients, LV diastolic pressures are normal at rest but become abnormal under exercise. The typical echocardiographic parameters acquired during exercise or immediately thereafter are the E/e’ ratio and peak tricuspid regurgitant velocity.14 These parameters have a high specificity (96 %) but a relatively low sensitivity (76 %). An increase in the E/e´ ratio under physical exercise indicates a concomitant increase in LV end-diastolic pressures and a worse prognosis.15,16
Strain rate during the isovolumetric relaxation time (IVRsr) or early diastolic strain rate (e´sr), derived from global longitudinal speckletracking strain, were recently proposed to estimate LV filling pressures, although both parameters are still experimental.17
Right Ventricular Function
Pulmonary artery systolic pressure and TAPSE represent the minimum dataset of RV parameters in HF patients.18 Other common techniques used are DTI derived s´ wave velocity, fractional area change and Tei index (which also evaluates diastolic function).
One emergent technique is RV 3D EF, which does not require geometric assumptions. Real-time 3D techniques have been shown to accurately provide objective measurement of RV volumes19 and are especially attractive after cardiac surgery, when conventional indices of longitudinal RV function are generally reduced.20
Strain, particularly speckle tracking technique of the free wall, is a promising technique. Although less validated than in the LV, pooled data suggest that global longitudinal RV free wall strain lower than -20 % is likely abnormal. This parameter has prognostic value in advanced chronic HF.21,22
Aetiology of Heart Failure
Echocardiography is inferior compared with other imaging methods (e.g. CMR) in determining the aetiology of HF. However, stress echocardiography can rule out ischaemia (with experimental techniques such as the use of microbubbles in development),23 and is of special interest because of its dynamic nature, in doubtful cases of hypertrophic cardiomyopathy or mitral regurgitation.24,25 Moreover, 3D echocardiography can help to elucidate the exact mechanism of some underlying valvulopathies, e.g. mitral valve prolapse.
Selection and Optimisation of Therapies
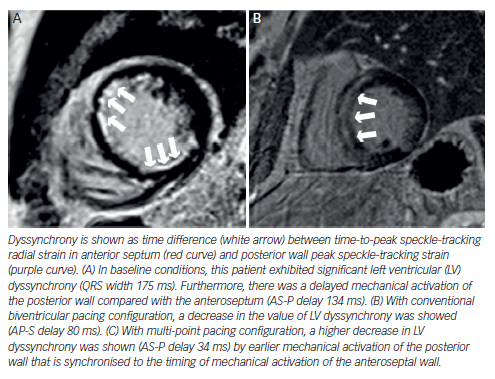
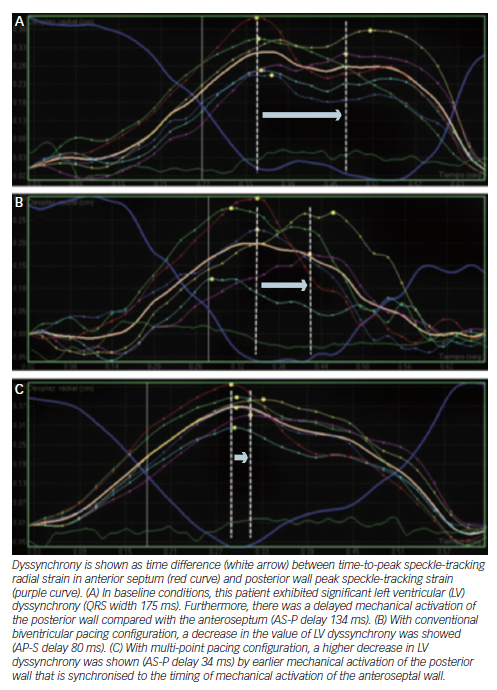
Echocardiography has been exhaustively evaluated in cardiac resynchronisation therapy (CRT), and some signs, e.g. septal flash, seem to indicate higher likelihood of success. Although dyssynchrony failed to improve patient selection beyond electrocardiogram (ECG) criteria,26 newer methods such as speckle tracking and 3D echocardiography are under investigation. By contrast, radial strain demonstrated its value in guiding the placement of the LV lead and subsequently improving the response to CRT in the small trials Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy (TARGET) and A Prospective Randomized Controlled Study of Echocardiographic-Guided Lead Placement For Cardiac Resynchronization Therapy (STARTER).27 Nowadays, no accepted echo criteria for CRT implantation exist, and it is still an experimental field.
Echocardiography also has a main role in the selection and follow-up of patients with LV assist devices, with routine exams recommended to optimise the performance of these devices.28
Cardiovascular Magnetic Resonance
The high spatial and temporal resolution of CMR makes it suitable for use in the assessment of right (RV) and LV, providing a comprehensive study that includes anatomical evaluation, functional data and great information about myocardial perfusion and viability.29
During a CMR examination, the patient is brought into a highstrength static magnetic field that aligns the spins of the hydrogen atoms. These atoms are then excited intermittently by pulses of radiofrequency waves (MR sequences) and the signal emitted from the body in return is detected, determining two distinct MR relaxation parameters, longitudinal relaxation time (T1) and transverse relaxation time (T2). A CMR sequence consists of a series of radiofrequency pulses, magnetic gradient field switches and timed data acquisitions. To prevent artifacts from cardiac motion, most CMR images are generated with fast sequences gated to the R-wave of the ECG. Respiratory motion, another source of artifacts, is usually eliminated by acquiring CMR images in end-expiratory breath-hold.
Late gadolinium enhancement (LGE) patterns have been shown to provide diagnostic utility for distinguishing between ischaemic cardiomyopathy (ICM) and non-ischaemic cardiomyopathy (NICM),30 but gadolinium-based contrast agents (GBCA) have been recently linked with a rare multisystemic fibrosing disorder known as nephrogenic systemic fibrosis. The patients at risk of developing this disease are those with severe renal insufficiency (glomerular filtration rate <30 ml/min/1.73 m2), there is no specific treatment and symptoms may appear from several days to few years after the exposition. Therefore, in high-risk patients, GBCA should be avoided unless the diagnostic information is essential and not available from non-contrast enhanced CMR or other imaging modalities
Ischaemia Assessment
CMR, and in particular the LGE and T2-weighted (‘edema’) imaging, is useful to determine whether a LV dysfunction has an ischaemic aetiology.31,32
A recent study on the diagnostic utility of CMR found 100 % sensitivity and 96 % specificity for the identification of the cardiomyopathy aetiology,33 reducing requirements of invasive angiography. However, total absence of LGE does not completely rule out ischaemic cardiomyopathy in the rare setting of global myocardial hibernation.
The typical LGE in ICM should always involve the subendocardium (subendocardial or transmural) and be located in a region that is consistent with the perfusion territory of an epicardial coronary artery.34 Between 10 % and 26 % of NICM patients without features of infarction show patchy or longitudinal striae of midwall hyperenhancement unrelated to a particular coronary artery territory, this distinct pattern of LGE corresponds to focal fibrosis.35
Stress perfusion CMR accurately identifies significant CAD, with higher accuracy than SPECT perfusion imaging.36 In HF patients, CMR has shown an excellent safety profile when assessing ischaemia or viability.37
Viability Assessment
Multiple studies have confirmed the ability of LGE-CMR to predict recovery of contractile function after revascularisation.38,39 Despite the contradictory findings of the Surgical Treatment for Ischemic Heart Failure (STICH) viability trial,40 a more recent study has shown that the identification of viable but dysfunctional myocardium using LGE-CMR in HF patients is associated with worse outcomes when managed medically rather than undergo surgical revascularisation.41
Scars taking up >75 % of wall thickness indicate that the likelihood for functional recovery is low, but scars <25 % have a positive predictive accuracy of functional improvement.42 The predictive value of segments with an intermediate (25 % to 75 %) transmural extent of scarring is lower. In these situations, a low-dose dobutamine study to assess the contractile reserve may be helpful.
A considerable number of patients (5–50 %) show lack of restoration of blood flow at myocardial level despite a successful procedure. This is called no-reflow and is due to microvascular obstruction.43 It is related to more severe myocardial damage, increases with the duration of ischaemia time and is independently associated with lack of functional recovery, adverse ventricular remodelling and worse patient outcome.44,45 It typically presents on LGE imaging as a subendocardially located hypointense area within the enhanced myocardium.
Therapy Guidance
The scar extent and the spatial distribution of LGE have been proposed as predictors of response in HF patients referred for CRT.46,47 Those patients with transmural necrosis in either the septal or inferolateral walls experience an absence of improvement in LV volumes at 6 months. Performance of LGE-CMR prior to CRT implantation can help to guide the procedure; therefore, the lead can be delivered to viable tissue targets, improving event-free survival.48
In the same way, LGE-CMR can also predict arrhythmia risk. Among patients with ICM or NICM, those having appropriate ICD therapy or who had survived sudden cardiac death (SCD) showed higher scar extent on LGE imaging.49
Diffuse Fibrosis Assessment
One of the most promising future applications of CMR is the ability to identify diffuse myocardial collagen content. This approach could be useful to detect subclinical disease in at-risk populations, such as hypertensive or diabetic patients, infiltrative myocardial diseases, etc. This technique is based in T1 mapping of pixel-based quantifications of gadolinium retention.50
Cardiovascular Magnetic Resonance in Other Cardiomyopathies
Acute myocarditis may present as new-onset HF and its diagnosis could be challenging. The T2-weighted images show hyperintense subepicardial and midwall areas of myocardial oedema.51 LGE is typically seen more pronounced in the subepicardial areas in inferolateral segments. Finally, hyperaemia and capillary leak (visualised on CMR images acquired early after gadolinium injection) have shown to be the best predictor in patients with chronic myocarditis.52
Regarding hyperthrophic cardiomyopathy, LGE extent is an independent predictor of adverse outcome.53,54 In cardiac amyloidosis, LGE characteristically involves the subendocardium in a circumferential pattern, showing sometimes a patchier transmural pattern.55
Nuclear Imaging
Nowadays, the main clinical application for radionuclide imaging in HF is myocardial perfusion imaging for the assessment of ischaemia and/or viability.56 The evaluation of sympathetic innervation by SPECT or PET has regained interest in the last years due to the apparition of new articles regarding its prognostic and predictive value.
Myocardial Blood Flow and Viability Testing
One of the first steps when studying a patient with HF is the identification of the underlying aetiology. Moreover, those patients with ICM and viable myocardium could benefit from revascularisation procedures, as some dysfunctional myocardium may not be irreversibly damaged. Nuclear imaging could be useful in this setting.
SPECT imaging with thallium-201 (201Tl) or technetium 99m (99mTc) has shown remarkable diagnostic capabilities to evaluate for the presence of infarction, ischaemia and/or viability.56 Prior studies suggest excellent negative predictive values but poor positive negative values.57 Reversible perfusion defects indicate ischaemia and fixed defects indicate scarring tissue. In general, ICM shows more extensive, diffuse and severe perfusion defects than NICM, but a noteworthy degree of overlap exists. Gated SPECT improves accuracy and provides information on LV volumes, LVEF and motion abnormalities.58 Summed scores can be derived from this technique. Higher summed stress scores were found in patients with ICM.58 SPECT perfusion defects also predict mortality in patients with ICM.59,60
PET imaging can also be used for this purpose. This technique determines an absolute quantification of myocardial blood flow and coronary flow reserve (CFR), as well as providing higher temporal and spatial resolution. For this reason, PET has better diagnostic performance than SPECT to detect ICM.61 In addition, PET myocardial perfusion reserve has shown61 to be a stronger predictor of unfavourable outcomes in ICM. Likewise, in NICM, CFR abnormalities can be detected due to microvasculature disease62 and have been associated with increased risk of death.63
Radionuclide techniques are of use also to evaluate the presence of viable myocardium in patients with ICM. SPECT has been widely utilised in this scope, demonstrating high sensitivity to detect viability.64,65 Nevertheless, the gold standard for myocardium viability assessing is 18-fludeoxyglucose (18F-FDG) PET. Metabolic PET imaging yields the highest accuracy (>90 % sensitivity) for predicting functional recovery66 after revascularisation. Data obtained from the PET and Recovery Following Revascularization-2 (PARR-2) study67 showed a threshold of 7 % of hibernating myocardium above which a patient could benefit from revascularisation. Although there is considerable evidence in the literature68 proving improved survival with revascularisation in patients with viable myocardium, surprisingly, the STICH study40 failed to find a correlation between demonstrations of myocardial viability and benefit from revascularisation.
Sympathetic Activity
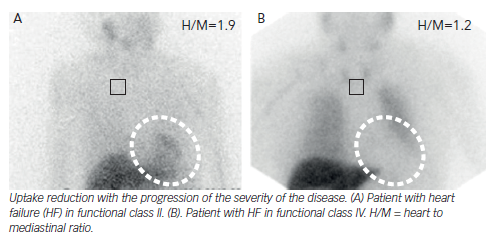
Hyperactivity of the autonomic nervous system (ANS) plays a major role in the pathophysiology of HF, causing myocardial β-adrenoceptor down-regulation. Viable myocardium with reduced innervation may be hyperresponsive to catecholamines, leading to the development of ventricular tachycardia (VT). There are SPECT/PET tracers that are analogues of the sympathetic neurotransmitters.69 As they are uptaken and stored in the presynaptic nerve endings, the visualisation of sympathetic innervation is warranted. SPECT imaging with iodine- 123-metaiodobenzylguanidine (123I-MIBG) is the most often used. 123I-MIBG studies comprise both planar and SPECT imaging in two phases: early (10–20 minutes after tracer administration) and late (3–4 hours after). Image analysis includes the heart mediastinal ratio (H/M) for the quantification of global uptake, the washout rate (WR) to reflect catecholamine turnover and sympathetic activity and regional uptake on SPECT imaging. In HF patients, cardiac innervation is reduced, thus 123I-MIBG uptake is globally reduced.
H/M has emerged as one of the most powerful independent predictors of adverse cardiovascular events.70,71 The AdreView Myocardial Imaging for Risk Evaluation in Heart Failure (ADMIRE-HF) trial,72 a prospective multicentre study involving 961 patients, showed that a H/M <1.6 doubled the risk of HF progression, VT or cardiac death. More recently, a Japanese meta-analysis including 1,322 patients73 proved that patients with H/M <1.68 or WR >43 % showed lower survival over a mean follow-up of 6.5 years. Other studies have evaluated 123I-MIBG imaging for the prediction of VT and SCD. In one of them, H/M resulted as an independent predictor for appropriate ICD therapy;74 while in the other one WR correlated with higher SCD occurrence.75 Moreover, studies consistently show that the H/R can reflect response to HF therapies, such as β-blockers76 or LVADs,77 as increases in H/R correlate with other clinical or analytical parameters.
Regional uptake assessment on SPECT adds relevant clinical information. Areas with autonomic tracer defect, but preserved perfusion tracer uptake (autonomic-perfusion mismatch), are more prone to develop lethal arrhythmias due to denervation supersensitivity.78 The extent or severity of autonomic defects predicted VT inducibility79 and occurrence of an ICD discharge or SCD.80
PET tracers such as 11C-hydroxyephedrine (11-CHED) improve the signal–noise ratio allowing better quantification.81 The recent Prediction of Arrhythmic Events with Positron Emission Tomography (PAREPET) study82 has proved that the extent of uptake defects correlated with the occurrence of SCD or ICD discharge.
Despite the increasing evidence, currently the assessment of cardiac innervation is not recommended as a routine test in the evaluation of HF patients. More studies are needed to define the subgroup of patients that could benefit most of this imaging technique.
Molecular Imaging
Molecular imaging techniques are emerging tools providing insight into disease manifestation before structural and physiological abnormalities become evident, although to date it has mainly been used for research purposes. A key factor in HF patients is myocardial remodelling. A multitude of cellular pathways implied in remodelling processes can be targeted: extracellular matrix degradation, angiogenesis, collagen deposition, etc.83,84 Most tracers are still under development and have been only tested in animal models. Imaging myocardial activity of renin–angiotensin system seems to be a promising tool to monitor disease progression and medical therapy effectiveness in HF patients.85
Computed Tomography
Latest generation of multidetector CT (MDCT) allows obtaining high-quality images using a smaller amount of iodinated contrast.86 In patients with HF, the increasing use of ICD-CRT devices, limits the possibilities of performing a CMR. In this group of patients, MDCT emerges as a valid alternative to assess LV volumes and function.
Coronary Artery Disease
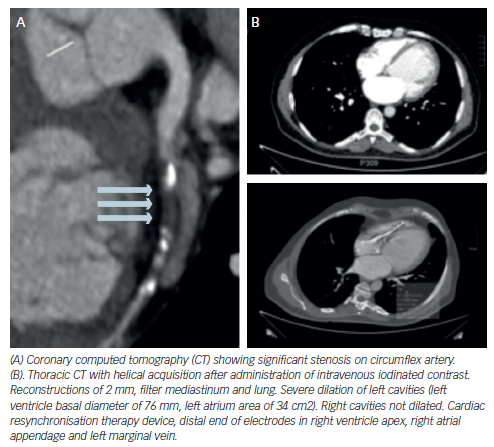
Evaluation of coronary calcium is a reliable test to discriminate ICM from NICM.87 Coronary MDCT in patients with HF has a high negative predictive value to confirm in a non-invasive way the absence of CAD,88–94 especially in new-onset HF patients. Taking into account the significance of CAD in HF patients, MDCT may be one of the most important non-invasive tests to perform in these patients. However, coronary MDCT provides anatomic but not physiological data on coronary disease, so no information about perfusion abnormalities can be extracted from it.
Due to the different attenuation characteristics of infarcted versus normal myocardium, measuring the infarct size is possible with MDCT. Those measurements have accurately correlated with those obtained on nuclear scanning and CMR.95–98
Cardiac Structure and Function
Several studies have shown excellent correlation between cardiac MDCT and other imaging modalities regarding diverse LV measurements: LVEF and global function, regional wall motion, wall thickness, chamber diameter, chamber volumes, stroke volume and cardiac output,99–103 and provides adequate visualisation of RV wall thickness and function.104,105 It can also obtain useful images to differentiate between some anatomic aetiologies of HF (for example, dilated versus hypertrophic cardiomyopathy). However, CMR is considered the gold standard for ventricular assessment and echocardiography is the most commonly used clinical test for this purpose.
Other Uses
MDCT can provide an accurate anatomic description of the pericardium (pericardial thickening, calcification, fatty infiltration and effusion), information of the anatomy of cardiac valves and assessment of ventricular contraction dyssynchrony, especially in those being assessed for CRT implantation.106
In cardiac transplantation, CT may obviate the need for routine invasive angiography to assess coronary allograft vasculopathy.107,108
Limitations and Contraindications of Computed Tomography
The two major risks with the procedure include contrast administration and radiation exposure. Cardiac CT cannot be performed in patients with contraindications to injection of iodinated contrast. Other relative contraindications include moderate to severe renal insufficiency and previous allergies to contrast. As for radiation, the effective radiation dose with 64-slice MDCT angiography is estimated to be approximately 11 to 22 mSv.
Hybrid Devices Computed Tomography Nuclear Image
Dual-image techniques offer the opportunity to use a single device for different purposes, such as determining perfusion, function and metabolism: adenosine stress CT myocardial perfusion imaging could detect haemodynamic significance of coronary stenosis detected by CT angiography.109,110 Diverse combinations of hybrid imaging of myocardial perfusion (CT + SPECT, CT + PET and CT + CMR) are gaining increasing interest because they provide both anatomic and functional information, improving the overall performance of the diagnostic test.
Conclusions
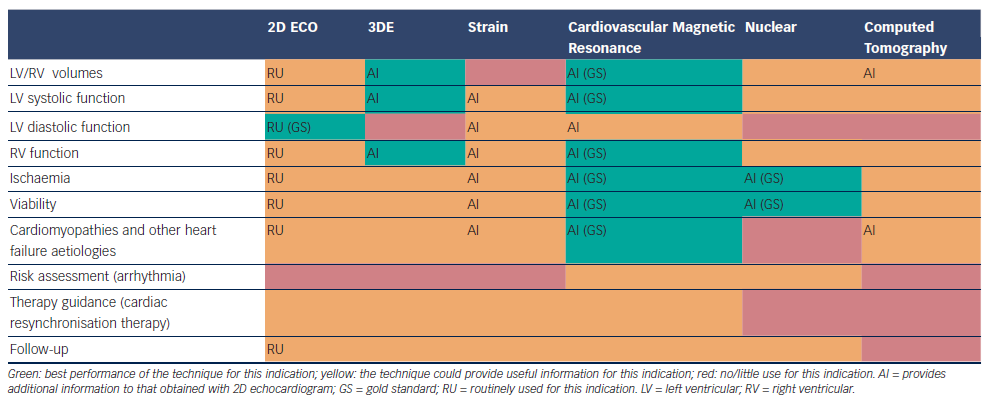
There are several imaging modalities available for the evaluation of HF patients, each one with its highlights and pitfalls. Echocardiography continues to be the method of choice for its availability, cost and usefulness, it provides most of the information required for the management and follow up of HF patients and it has been enhanced with the development of 3DE and strain. Other non-invasive cardiac imaging modalities could provide additional aetiological, prognostic and therapeutic information, being helpful in making treatment decisions, especially in some subsets of patients (ischaemic heart disease, cardiomyopathies …) (see Table 1). An appropriate utilisation of imaging procedures should improve management and clinical outcomes in HF patients.