Right ventricular (RV) failure is associated with significant morbidity and mortality, with in-hospital mortality rates estimated as high as 70–75%.1–3 RV failure may occur following cardiac surgery, in conjunction with left ventricular (LV) failure (e.g. in acute decompensated heart failure), or isolated in circumstances, such as inferior MI with RV infarction, pulmonary embolism (PE) or following left ventricular assist device (LVAD) placement.4–10
Medical management includes volume optimisation, inotropic therapy and vasopressor support; a subset of patients may benefit from mechanical circulatory support (MCS) for persistent RV failure.9,11,12 Increasingly, percutaneous and surgical mechanical support devices are being used for RV failure.1,13–15
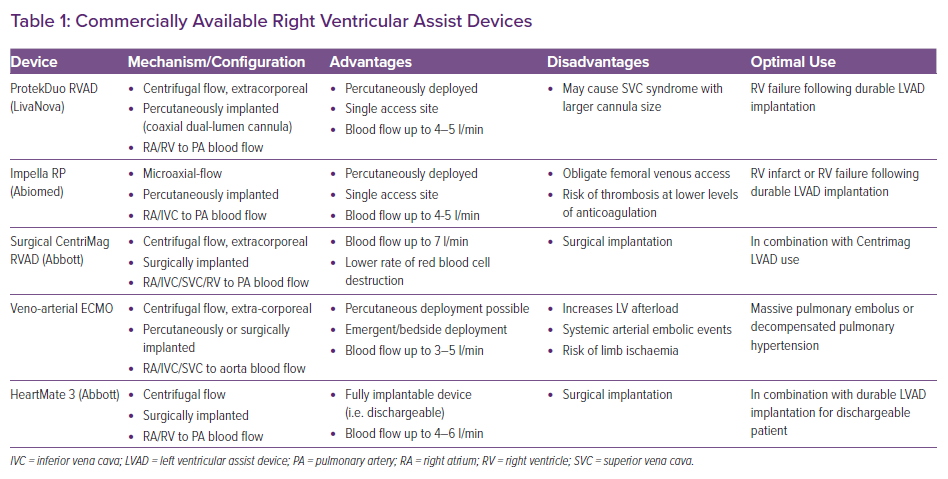
Devices for isolated RV support include percutaneous options, such as micro-axial flow pumps and extracorporeal centrifugal flow right ventricular assist devices (RVADs), surgically implanted RVADs and veno-arterial extracorporeal membrane oxygenators (VA-ECMO). In this review, we will discuss the indications, candidate selection, strategies and outcomes of MCS for RV failure.
Pathophysiology
The primary mechanisms of cardiogenic shock secondary to RV failure include pump failure, volume overload and pressure overload.9 Pump failure leads to a reduction in contractility in the setting of primary myocardial injury (e.g. myocarditis or RV ischaemia). A decreased stroke volume leads to dilation of the RV. This exacerbates tricuspid regurgitation, which may lead to further RV dilation.9
Volume overload can also lead to RV failure. A typical example of this is RV failure following LVAD implantation. When the left ventricle (LV) is unloaded with an LVAD, there is increased venous return to the right side of the heart, which can worsen pre-existing RV failure.16–20 This may be exacerbated by altered position of the interventricular septum, resulting in diminished RV stroke volume. Finally, RV pressure overload may result from decompensated left-sided heart failure, pulmonary hypertension or acute PE.14,21
Medical therapy often involves optimisation of preload with volume expansion or diuretic therapy, reduction of afterload with pulmonary vasodilators and inotropic therapy.9,11 However, the main focus of this review will be on MCS options for patients who have RV failure refractory to medical therapy.
A reason for optimism regarding MCS options for the RV arises from the ability of the RV to recover from various insults relatively quickly. This makes it an attractive target for short-term circulatory support devices. For example, because it has a lower myocardial oxygen demand than the LV, the RV often recovers from ischaemic insults following an acute coronary syndrome.22 In addition, while some patients will experience RV failure after LVAD implantation and require RVAD implantation, interventions designed to improve RV performance often allow for timely wean from these short-term devices.
Patient and Device Selection
Given the availability of both percutaneous and more invasive surgical options, an interdisciplinary approach is necessary when choosing the most appropriate therapy for each patient.23–25 Vital perspectives are provided from shock team, including from advanced heart failure specialists, interventional cardiologists, cardiac surgeons and intensive care physicians.
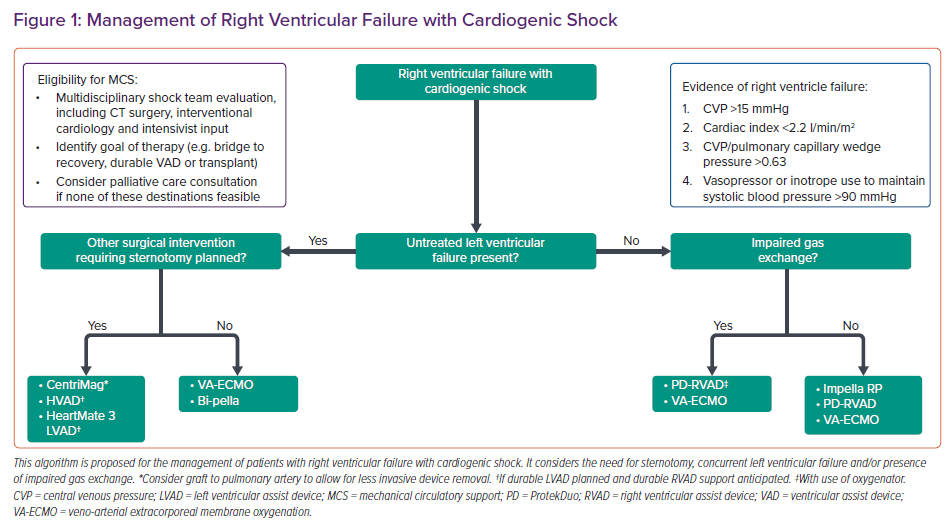
Patients should be identified early to avoid potentially irreversible end-organ injury. The choice of device will depend on whether the underlying process is a primary RV insult, valvular pathology or biventricular insult (Table 1).9 Considerations include the haemodynamic impact of the device and technical aspects, as well as the exit strategy for these patients, including their candidacy for durable ventricular assist devices and organ transplantation (Figure 1).
Percutaneous Mechanical Support Devices
Intra-aortic Balloon Pump
Intra-aortic balloon pumps (IABPs) are commonly employed in LV failure due to MI or cardiomyopathy. However, they are less effective in situations of acute RV failure. IABPs help to reduce LV afterload. By unloading the LV, they may reduce right-sided filling pressures and/or increase right coronary perfusion, but these effects are indirect.1 However, studies have shown minimal haemodynamic benefit, especially in RV failure, and suggest many patients will require escalation of mechanical support.26,27
Microaxial Flow Transvalvular RVAD
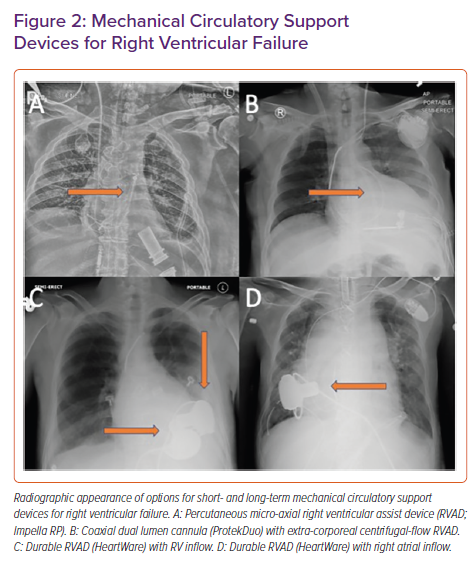
The Impella RP (Abiomed) is a micro-axial pump that can be inserted percutaneously via the femoral vein. The pump head is 23 Fr and is mounted on an 11 Fr catheter. It provides up to 5 l/min of flow and is approved for use for up to 14 days.1 When it is in the correct position, blood is drawn into the pump from the inferior vena cava-right atrial junction and ejected into the main pulmonary artery (PA). Its appearance on chest radiography is shown in Figure 2, along with other RV support devices.
In the RECOVER RIGHT study, 30 patients with refractory right heart failure prospectively received the Impella RP device. Approximately half of the cohort had developed RV failure following LVAD implantation while the remaining patients had RV failure following cardiotomy or MI.28 A follow-up study ultimately expanded this cohort to 60 patients.29 Haemodynamics improved rapidly with an increase in cardiac index and a decrease in central venous pressure.
In 2019, the Food and Drug Administration (FDA) warned about the increased mortality observed in patients supported by the device. This was likely due to use of the device outside the indications described and the severity of illness of patients supported by it. An interim analysis of the post-approval study showed that the survival rate for the patients who would have met the enrollment criteria for the clinical trials was 72.7%, which is similar to the survival rate in the premarket clinical study (73.3%).30
The Impella RP has also shown beneficial haemodynamic effects in patients with acute RV failure in the setting of PE.31,32 In patients who were refractory to volume expansion and inotropic support due to a massive or submassive PE, support with the Impella RP device lowered mean heart rate, increased mean systolic blood pressure and improved the cardiac index.31
During the COVID-19 pandemic, the FDA issued an emergency use authorisation for Impella RP for patients experiencing RV failure or decompensation due to complications of COVID-19 infection,
including PE.33
The Impella RP should be used with caution in patients with tricuspid valve regurgitation. According to the manufacturer, tricuspid valve regurgitation is a contraindication. However, functional tricuspid regurgitation caused by dilation of the valvular annulus may improve with Impella RP treatment.34 Pulmonary regurgitation, however, is a major contraindication for the use of this device.
A significant advantage of the Impella RP is its need for only a single venous access site as well as its percutaneous placement, although only femoral access is possible. Haemolysis has been reported for other Impella devices but less is known about its incidence with Impella RP.
Extra-corporeal Centrifugal Flow Percutaneous RVAD
This device configuration employs an extracorporeal centrifugal-flow pump (e.g. TandemHeart [LivaNova] or CentriMag [Abbott]) with percutaneous venous cannulation that withdraws blood from the right atrium and ejects into the main PA.14 An example of this is the TandemHeart used with the ProtekDuo cannula (LivaNova). Cannulation may be from bilateral femoral venous access, internal jugular access (if the ProtekDuo cannula is used) or a combination of the two sites. This percutaneous configuration has been employed in a variety of scenarios including MI, severe pulmonary hypertension, severe mitral regurgitation, allograft failure following heart transplantation and post-LVAD implant.14,35–39
The THRIVE registry studied 46 patients receiving a TandemHeart RVAD in eight centres.40 The TandemHeart RVAD was used in myocarditis, MI and chronic left heart failure, and following valve surgery, coronary artery bypass grafting, orthotopic heart transplant and LVAD implantation. Within 48 hours of RVAD deployment, haemodynamics, including mean arterial pressure, right atrial pressure, PA systolic pressure and cardiac index, were all significantly improved.
More recently, the ProtekDuo cannula has allowed percutaneous RVAD support to be established with a single venous access cannulation. The ProtekDuo cannula is a dual-lumen cannula that can be placed via the jugular vein and may be positioned in such a way that its distal port enters the PA. When used with an extracorporeal centrifugal blood pump, it can deliver blood from the right atrium to the main PA.41 It is capable of providing 4–5 l/min of flow and allows for ambulation given the lack of femoral cannulation.
In one dual-centre experience, involving 17 patients with RV failure supported by ProtekDuo-RVAD, 23% of patients were successfully weaned.42 However, more than 40% of patients died even with adequate pump flow. Twelve of these patients already had a durable LVAD in place. The benefits of this device configuration include the avoidance of sternotomy, particularly in patients who may have had prior surgery or may be transplant candidates. In certain cases, these devices have been used pre-emptively for RV support in patients undergoing durable LVAD implantation.43
An analysis at our centre compared 19 patients with percutaneous RVADs (both Impella RP and ProtekDuo-RVAD) with 21 patients with surgical RVADs.44 Both percutaneous and surgical support systems provided immediate improvements in haemodynamic profiles despite higher overall flows with surgical RVADs. In addition, percutaneous RVAD use was associated with less morbidity including decreased blood transfusion requirement and a shorter time being mechanically ventilated.
Surgically Implanted Support Devices
CentriMag
The CentriMag (Abbott) is an extracorporeal centrifugal pump that is approved for use as an isolated RVAD for up to 30 days in patients with cardiogenic shock.45 It has also been used as part of an ECMO circuit.46 It lacks mechanical bearings or seals, and its magnetically levitated rotor is thought to reduce blood trauma and mechanical failure.47 The device can be used as an RVAD with inflow and outflow cannulas. The inflow cannula may be positioned in the right atrium through direct insertion via the superior vena cava (or internal jugular, for example) or the inferior vena cava (or femoral vein, for example); alternatively, it may be inserted directly into the RV. The outflow is typically anastomosed to the PA, though reports have included connection through a graft sewn to the PA which allows the RVAD to be removed without reopening the chest.48 For patients with concomitant respiratory failure, an oxygenator may be added to the configuration.
A meta-analysis of 999 patients supported with the CentriMag found that it was used as a ventricular assist device in 72% of cases and as part of an ECMO circuit in 25%.46 Those included had experienced post-cardiotomy shock, post-transplant allograft rejection, RV failure following LVAD placement, as well as some pre-cardiotomy states. At 30 days, survival was 66% in pre-cardiotomy cardiogenic shock, 61% in post-LVAD placement, 54% in post-transplant allograft failure and 41% in post-cardiotomy cardiogenic shock.46
Biventricular Support Strategies
Surgical Biventricular Assist Device
Full biventricular support can be established with the use of a centrifugal flow extracorporeal pump, such as CentriMag used as an RVAD (described above), or in combination with an extra-corporeal LVAD configuration (typically with cannulation of the LV and aorta). Such a configuration may provide up to 7 l/min of circulatory support with full unloading of both ventricles.
Percutaneous Biventricular Assist Device
The use of the Impella RP device in combination with a percutaneous LVAD from the same manufacturer has been reported in patients with biventricular failure.49–52 The degree of circulatory support with this configuration depends on the maximum flow provided by the percutaneous LVAD, which is in the range of 3.5–5 l/min.
Extracorporeal Membrane Oxygenation
VA-ECMO has become an increasingly used method of short-term haemodynamic support in cardiogenic shock.53 It simultaneously provides extracorporeal gas exchange and circulatory support in the setting of left, right or biventricular failure.54 The circuit consists of a venous inflow cannula, centrifugal flow pump, oxygenator, heat exchanger and outflow arterial cannula. VA-ECMO can be employed centrally or with peripheral access (e.g. by the femoral vein and artery).
Typically, central VA-ECMO is used in patients unable to be weaned from cardiopulmonary bypass whereas peripheral VA-ECMO can be initiated percutaneously.54,55 It has become increasingly used specifically in cases of fulminant myocarditis, allograft failure after cardiac transplantation, acute RV failure due to PE, RV failure during LVAD support and severe decompensated heart failure.53,56–61 It is important that these patients have an exit strategy, which may include bridge to recovery, durable LVAD or heart transplantation.
VA-ECMO can provide 3–5 l/min of flow depending on cannula size. Since it drains blood directly from the central venous system, it decreases RV preload and therefore can be helpful in cases of RV failure secondary to volume and pressure overload. A distinction should be made, however: while VA ECMO provides circulatory support irrespective of RV or LV function, it differs from a traditional RVAD in that it establishes a parallel circulation as opposed to being an actual ventricular assist device. Because of this, when used for RV support after LVAD implantation, VA-ECMO decreases flow through the LVAD, potentially increasing the risk of device thrombosis.
One disadvantage of VA-ECMO is the increase in afterload with the potential for LV distension and overload.62 The increase in left atrial pressure can induce or worsen pulmonary oedema and lead to stasis within the LV and aortic root.54 Therefore, many clinicians will initiate a ‘venting’ strategy to prevent the complications of LV pressure overload. Options include percutaneous LVAD, such as Impella, IABP, atrial septostomy or direct cannulation of the left atrium or LV.54
A minimally invasive surgical approach combining an extracorporeal LVAD with extracorporeal membrane oxygenation (Ec-VAD) for short-term biventricular circulatory support has been used as a bridge to durable LVAD or recovery.63,64 A minithoracotomy is performed for direct LV apical cannulation, which is combined with femoral venous inflow and outflow cannulation of the right or left axillary artery. Compared to conventional extracorporeal surgical LVAD implantation, Ec-VAD patients have shorter cardiopulmonary bypass times and significantly lower incidences of bleeding events with similar flow rates. The 30-day survival was similar between groups.63
Other potential complications of peripheral VA-ECMO include lower extremity ischaemia, which has been shown to occur in 12–22% of patients.65 To obviate this risk, a 6–8 Fr vascular introducer can be placed to provide antegrade distal perfusion to the cannulated extremity. In addition, roughly 25% of all VA-ECMO patients have major bleeding complications.66 This can occur even in patients who are not on anticoagulation therapy.54 Bleeding complications may be reduced by the use of smaller arterial cannulas.67
Durable Biventricular Assist Devices
A significant proportion of individuals require RV MCS following durable LVAD placement and fewer than half of these patients can be weaned from temporary RVAD support.68,69 Therefore, various strategies of durable biventricular support have been employed and described.68,70–72 According to the INTERMACS registry, 618 durable continuous-flow BiVAD procedures have been performed.73
Shebab et al. have described the use of the HeartWare ventricular assist device (HVAD; Medtronic) as a biventricular assist device for patients awaiting cardiac transplantation.71 Six patients underwent right HVAD implantation in the RV free wall while seven patients had it implanted in the RA free wall. RVAD pump thrombosis occurred in three of six RV pumps and one of seven RA pumps. This series demonstrates one of the difficulties in using assist devices in the RV; the heavily trabeculated RV and dense tricuspid subvalvular apparatus can predispose patients to suction events. Implantation in the RA may be more favourable.68
In another series, 11 patients with biventricular failure underwent implantation of an LVAD as well as an HVAD in the RA.68 Still, pump thrombosis occurred in four patients, who required treatment with bivalirudin and cannula-directed tissue plasminogen activator.68 One reason for the elevated incidence of device thrombosis may be related to the need to maintain lower pump speeds to avoid generating excessive flow through the low-resistance vascular bed. Of note, in August 2021, because of increasing incidences of adverse neurological events and pump thrombosis, the FDA issued a class I recall for the HeartWare HVAD system.74
More recently, the HeartMate 3 (Abbott) has been used in a biventricular configuration.75 Given the low incidence of thrombosis recorded with the HeartMate 3, it is an appealing device to use in the highly trabeculated RV.76 In the first experience described, which involved 14 patients, eight patients underwent simultaneous RVAD and LVAD implant while the others underwent RVAD implantation following LVAD implant.75 The RVAD was implanted into the RA in 12 patients. Nine patients were still alive at the time of publication.
McGiffin et al. also describe 12 patients who underwent similar biventricular HeartMate 3 implantation as a bridge to cardiac transplantation.77 The right-sided pump was implanted in the right atrium. Three cases of right VAD thrombosis were reported: one was managed medically, one required surgical pump exchange and one was intraoperatively treated with clot retrieval. By 18 months after implantation, five patients had undergone cardiac transplantation, five were alive on biventricular support, one had died and one had the VAD explanted for myocardial recovery.
Future Directions
PERkutane KATheterpumptechnologie RV (PERKAT RV, NovaPump) is a newer device, designed with the aim of creating a minimally invasive mechanical right heart support device that modifies the pulsatile support technology of IABP therapy. It is meant for rapid percutaneous deployment requiring an 18 Fr sheath. The device is composed of a nitinol chamber covered by foil that contains inflow valves.
The chamber is implanted in the inferior vena cava and the outlet tube attached to its distal part has its tip in the pulmonary trunk, bypassing the right heart.78 An IABP balloon is then placed inside so that, during balloon inflation, blood flows into the pulmonary arteries. The device has been shown to achieve flow rates of 3.5 litres per min in vitro. In a sheep model of acute pulmonary embolism, the device increased cardiac output by 59%.79 However, future studies are needed to determine its efficacy and outcomes in humans.
Gaps in Knowledge
While different mechanical support platforms hold great potential for improving patient outcomes related to RV failure, it is important to acknowledge the absence of randomised trial data to guide the use of this technology. Furthermore, the difference between outcomes with the Impella RP device in a study population and the post-market experience highlights the importance of careful patient selection and the need for more high-quality data to support the use of these technologies.
While the focus of durable RVAD investigation has been on patients with RV failure following durable LVAD implantation, interest is growing in the use of isolated durable RVAD use for patients with other disease processes that typically affect the RV and spare the LV. HVAD use has been reported in isolated RV failure secondary to WHO group 1 pulmonary hypertension when lung transplantation is not feasible.80
In addition, the optimal use of durable RVADs for patients with durable LVADs remains unclear. The optimal timing of percutaneous RVAD insertion for patients at high risk of RV failure following LVAD insertion is unknown, with some centres initiating RVAD support before implanting an LVAD. Lastly, the relative benefit of one short-term RV MCS device over another is also unclear and may vary according to the underlying aetiology of RV failure.
Conclusion
RV failure portends a poor prognosis across a spectrum of cardiovascular disease states including RV infarction and post-cardiotomy shock as well as following LVAD implantation, among other situations.
The ability of the RV to recover from a variety of pathophysiologic insults makes it an attractive target for short-term circulatory support devices. Recent advances in percutaneous therapies for short-term RV circulatory support offer promise to improve upon these historically poor outcomes. However, the long-term use of RV MCS devices remains limited, and outcomes are variable. Early recognition of RV failure and implementation of RV MCS devices are important steps to optimising outcomes for this patient population.