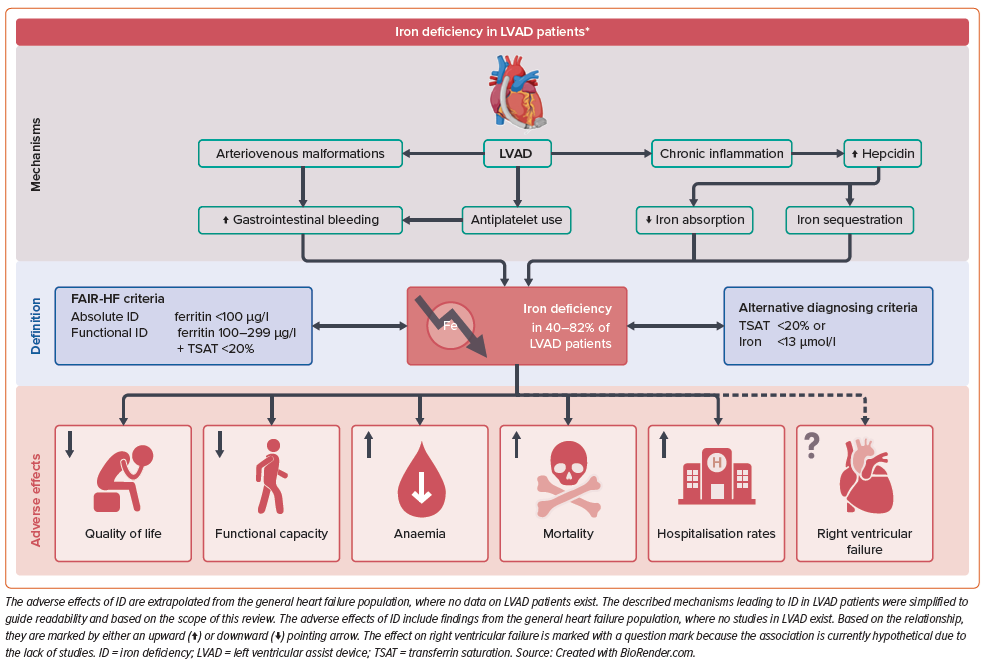
Iron deficiency (ID) is a common comorbidity in patients with heart failure (HF), in which iron stores are insufficient to meet the demands of the body for critical physiological functions, including oxygen storage and transportation (haemo- and myoglobin), mitochondrial oxidative metabolic processes and the metabolism of macronutrients as well as nucleic acids.1 ID in HF patients is associated with worsening symptoms, impaired functional capacity, reduced quality of life (QoL) and higher risks of mortality and hospitalisation, independent of anaemia.2–6
In patients with HF, ID is usually defined according to the FAIR-HF trial criteria as either absolute (ferritin <100 µg/l) or functional (ferritin 100–299 µg/l and transferrin saturation [TSAT] <20%).7 Using this definition, the prevalence of ID in HF patients is approximately 50%.2 The underlying pathophysiology of ID in the context of HF is multifactorial and not fully understood; it involves impaired intestinal function and hepcidin dysregulation due to the inflammatory state in HF. For the same reasons, oral iron supplementation is usually ineffective.8 IV iron repletion is a safe and viable treatment of ID in patients with HF, improving symptoms, QoL and exercise capacity.9 The effects of IV iron repletion on mortality and HF hospitalisations are less clear. A recent meta-analysis concluded that, compared with placebo, treatment with IV iron significantly reduced the composite outcome of recurrent HF hospitalisations and cardiovascular death (RR 0.77; 95% CI [0.66–0.90]; p<0.001).2 However, a recent large randomised trial, not included in the meta-analysis, did not find similar clinical benefit of IV iron in HF patients with ID.10
In patients with end-stage advanced HF, treatment options include heart transplantation and implantation of left ventricular assist devices (LVADs). Worsening shortages in donor organ availability severely limit heart transplantation, making LVADs an increasingly important alternative.11 Survival after LVAD implantation has improved in recent years, with 5-year overall survival at 58% with contemporary devices (HeartMate 3), as reported by the MOMENTUM 3 trial, and 2-year survival equivalent to that of heart transplantation recipients.12,13 Although LVAD therapy ameliorates HF symptoms and end-organ dysfunction and is associated with improvements in the 6-minute walk distance (6MWD) and QoL, exercise capacity (peak oxygen uptake; pV̇O2) remains significantly reduced after implantation.14,15 Furthermore, readmission rates are significant, at 1.3–2.6 per patient-year, attributable to episodes of infections, bleeding and HF-related events.15,16 It may be hypothesised that ID is an important mediator of these adverse events. In the recent updates of the European Society of Cardiology (ESC) guidelines on managing HF, treating ID with IV iron to improve QoL and functional capacity has been given a Class I recommendation.17 However, the guidelines do not address HF patients treated with LVAD. As such, there is uncertainty in the clinical community about how to manage ID in LVAD patients.
In this narrative review, we present an overview of the existing research on the prevalence, characteristics and effects of ID in the LVAD population, shedding light on potential implications for patient management and identifying areas for further investigation.
Methods
Search Strategy
A systematic literature search was conducted through the PubMed and Embase databases up to 26 September 2023 to identify ID studies in adult patients implanted with an LVAD (search terms are provided in Supplementary Table 1). Only original articles on ID in adult LVAD patients were included. After removing duplicates, screening was performed by title, abstract and full-text (Supplementary Figure 1). Ultimately, we searched ClinicalTrials.gov for ongoing trials, although we could only identify a single terminated study (NCT03774615).
Data Collection and Analysis
Study characteristics (e.g. centre, population size, inclusion/exclusion criteria) and patient baseline criteria (e.g. age, sex, LVAD type) were extracted from the published articles. Laboratory results were harmonised to SI units (e.g. creatinine in µg/dl to µmol/l) and durations of LVAD support were converted to months to facilitate comparisons. Continuous variables are presented as the mean with 95% CI or median with interquartile range (IQR), depending on the source material. Two-tailed p≤0.05 was considered statistically significant. Figure 1 was drawn using BioRender.com, whereas all statistical analyses and other visualisations were conducted in R.
Results and Discussion
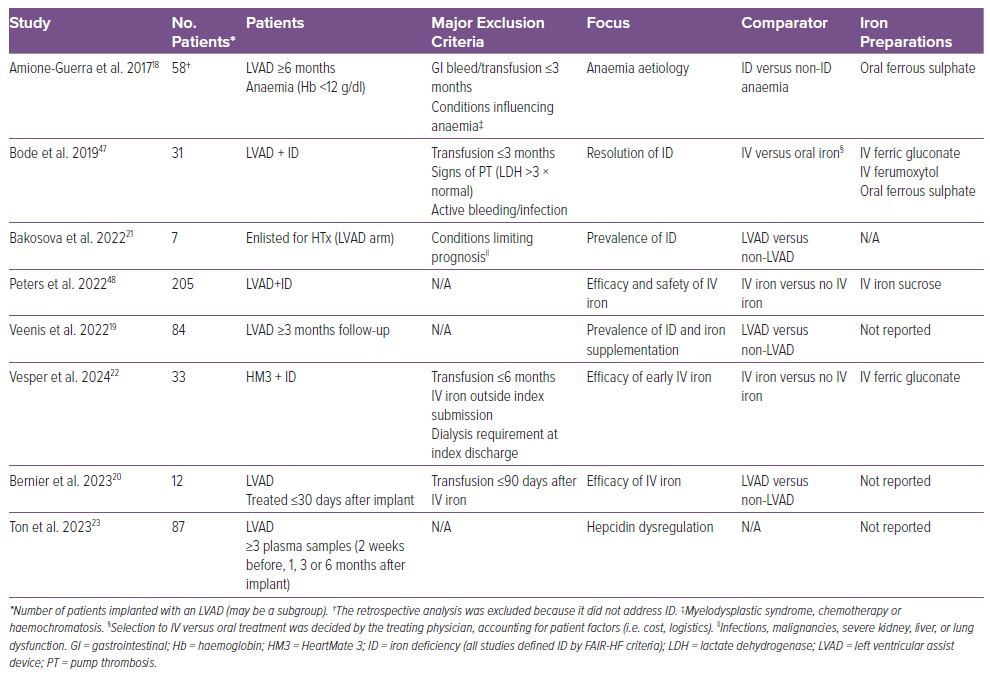
Our literature search identified eight studies of ID in LVAD patients, published between 2017 and 2023, with a combined population of 517 patients (Table 1). Seven studies were retrospective (one cross-sectional); all were single-centre studies and generally had small sample sizes. All studies used the FAIR-HF definition of ID, and iron supplementation was addressed in six studies.19,20,22,23,47,48 The study populations were similar in age, sex and HF aetiology, but differed in LVAD characteristics and the research area in focus (Supplementary Table 2). Across studies, the most common device was HeartMate 3 (49%), followed by HeartMate II (34%) and the HeartWare Ventricular Assist Device (14%), with the type of device unreported in 3% of patients. Key findings and concepts from our review are summarised in Figure 1.
How Common is Iron Deficiency in LVAD Patients?
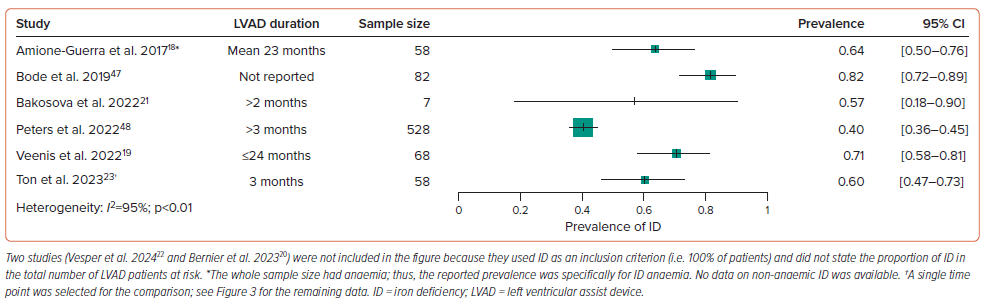
Prevalence data were reported in six studies and are presented in Figure 2, with prevalence ranging between 40% and 82%.18,19,21,23,47,48 Peters et al. identified ID in 213 of a total of 528 LVAD patients, making the prevalence of 40% notably lower than in the other studies.48 The authors of that study did not state whether all 528 patients were screened, which may explain the lower prevalence of ID. Absolute ID (ferritin <100 µg/l) was the most frequent subtype (~75%), and most patients had concurrent anaemia (67–87%).18–22
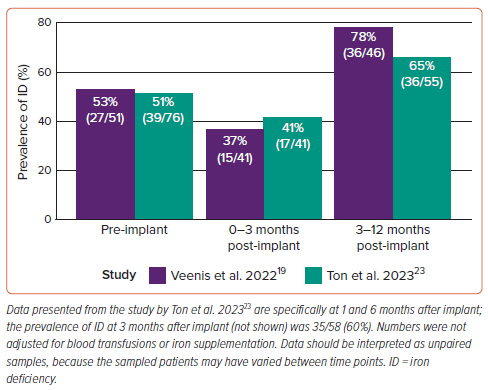
The prevalence of ID at different times (Figure 3) was reported in two studies, with a pre-implant (i.e. prior to LVAD implantation) prevalence of 53% and 51%, similar to the general HF population.2,19,23 The prevalence of ID decreased in the early post-implant period (0–3 months), but increased above baseline from 3 months onward, which may be explained by the peri- and postoperative blood transfusions related to LVAD implantation.24 Ton et al. reported that 79% of patients were transfused with a median of 3 units (IQR 2–6 units) of packed red blood cells <14 days after implantation.23 At subsequent follow-ups, the prevalence of ID increased even though haemoglobin levels increased.23 Transfusion rates were not reported by Veenis et al.19
Although all studies used the same definition of ID, the timing of iron measurements (relative to the time of implant) varied considerably across studies (e.g. both before index discharge and 2 years after implant). Criteria for when to test for ID (iron indices) were generally undisclosed by the studies. It is probable that adverse events, including bleeding and haemolysis, triggered blood testing, as in the study by Veenis et al., introducing a significant risk of sampling bias in the results.19 Interestingly, Veenis et al. reported that the number of patients screened for ID prior to LVAD implant increased significantly after the publication of the 2016 ESC HF guidelines (from 36% to 79% of patients screened; p<0.001), which may have influenced other studies as well.19,25 Although these numbers were not adjusted for blood transfusions or iron supplementation and were subject to further sampling bias due to the independent nature of the sampling, the results suggest that LVAD therapy does not alleviate ID. Protocolised testing for ID is necessary to estimate the true prevalence of ID in the LVAD population.
Defining and Diagnosing Iron Deficiency
The FAIR-HF trial definition of ID is widely used in HF research and was adopted by both American and European HF guidelines, but the accuracy of the criteria has been questioned. Ferritin is closely correlated to iron stores in healthy subjects, but acts as an acute phase protein, and thus levels increase during infections and other inflammatory conditions.25–27 Furthermore, chronic inflammation induces the hepatic hormone hepcidin, which inhibits iron absorption in the gut while increasing the release of ferritin from cells, in turn clouding the relationship between serum ferritin and iron stores in vivo.28 Taking this into account, the conventional definition used higher limits of ferritin to account for the chronic inflammation of HF, inspired by data on ID in patients with chronic kidney disease.28 Although the chosen cut-off values are empirical, they have successfully identified patients who benefitted from treatment with IV iron in several studies.9 TSAT, the quotient of serum iron divided by serum transferrin (or total iron-binding capacity), is a more direct marker of available iron in the bloodstream and is less sensitive to inflammation.29
To validate the FAIR-HF definition, Grote Beverborg et al. referenced serum iron indices to bone marrow iron staining, the gold standard for diagnosing ID.30 The FAIR-HF criteria had a sensitivity of 82% and specificity of 72% in diagnosing ID, and were surpassed by both TSAT ≤19.8% and serum iron ≤13 µmol/l (94% sensitivity for both and 84% and 88% specificity, respectively; p<0.05 versus the standard criteria).30 These alternative criteria have significantly outperformed the conventional definition in retrospectively identifying patients who benefitted from iron repletion across several HF cohorts.30–34
In a cohort of 387 HF patients, both TSAT and serum iron were significantly associated with mortality, whereas isolated hypoferritinaemia (ferritin <100 µg/l) did not predict mortality.30 When applying the results to a meta-analysis by Anker et al., patients with TSAT ≤19.8% had significantly improved outcomes (in terms of cardiovascular hospitalisation and cardiovascular death), whereas patients with TSAT >19.8% did not benefit from treatment with IV ferric carboxymaltose.30,31 Similarly, subgroup analysis in the iron-CRT trial showed a significantly greater effect of ferric carboxymaltose in patients with TSAT <20% (versus TSAT ≥20%).35 TSAT <20% predicted all-cause mortality in patients with HF with preserved ejection fraction and higher all-cause 5-year mortality, but FAIR-HF criteria did not.33,34 Curiously, Masini et al. found a trend towards lower 5-year mortality in patients with ferritin <100 µg/l (absolute ID; HR 0.91; 95% CI [0.81–1.01]; p=0.09). The AFFIRM-AHF trial did not find a treatment interaction with low versus high TSAT.34,36 Dhaliwal and Kalogeropoulos argued that the optimal definition depends on the outcome of interest. TSAT seems to be the superior predictor of mortality, whereas ferritin is a stronger predictor of functional capacity and QoL.37 Soluble transferrin receptor and hepcidin have been suggested as alternative and possibly more robust biomarkers of ID, but these are less readily available for routine testing.38
In the study by Ton et al., median ferritin levels initially increased from 188 µg/l (IQR 111–417 µg/l) before implantation to 320 µg/l (IQR 134–503 µg/l) at 1 month before decreasing to 91 µg/l (IQR 40–179 µg/l) 6 months after implantation (p=0.0001).23 Although rates of ID increased above baseline after 3 months, the median hepcidin/TSAT ratio decreased quantitatively from 103 ng/ml/% before implantation to 56 ng/ml/% 6 months after implantation (p=0.1), remaining above the 50 ng/ml/% limit of responsiveness to oral iron repletion established by the IRONOUT HF trial.23,39 The hepcidin/TSAT ratios were higher in patients with functional versus absolute ID and were not correlated with infections or bleeding episodes.23 It has been argued that LVAD therapy accentuates the inflammation associated with HF, at least initially, due to the shear stress and foreign body surfaces introduced by the device.40,41 Furthermore, driveline and other LVAD-related infections are common adverse events during LVAD therapy, which has been linked to a systemic inflammatory response, including interactions with the bone marrow, often undetectable by changes in serum concentrations of white blood cells or C-reactive protein.12,42,43 Fluctuations in the proinflammatory cytokine interleukin-6, a well-known inducer of hepcidin, are of interest, but reported changes during LVAD therapy have been conflicting.41,44 To the best of our knowledge, no studies have examined correlations between inflammatory and ID markers in LVAD patients.
Based on the reviewed studies of LVAD patients, ID is most often of the absolute type, characterised by depleted iron stores, usually ascribed to malnutrition, impaired gut absorption and chronic blood loss, but not inflammation.45 Gastrointestinal (GI) bleeding is a common adverse event of LVAD therapy: 5-year outcome data from the MOMENTUM 3 trial revealed that bleeding events have become less frequent in patients with contemporary HeartMate 3 devices, but remain substantial at 0.43 events per patient-year.46 Haemolysis events were minor at 0.0005 per patient-year.12 To adjust for this, several of the included studies excluded patients with recent GI bleeding (Table 1), but may have missed those with occult bleeding. The intricate and inverse effects of persistent blood loss and chronic inflammation on ferritin levels may further diminish the validity of the FAIR-HF criteria in LVAD patients. The optimal diagnostic criteria for ID in patients with LVAD remain uncertain; future studies validating the various definitions through bone marrow staining in LVAD patients would be beneficial.
Efficacy and Safety of Iron Supplementation
In total, 130 (25%) patients were treated with a single infusion of IV iron (iron sucrose, ferric gluconate, ferumoxytol or unreported), 131 (25%) were treated with oral iron (ferrous sulfate or unreported) and 36 (7%) were treated with both IV and oral iron. The decision to treat patients with iron supplementation was not randomised, and the studies generally did not disclose the indication criteria, exposing them to considerable risk of selection bias.
Both oral and IV iron treatment led to significant and comparable improvements in haemoglobin levels, ranging between +0.74 and 1.37 mmol/l at follow-up.18,20,47,48 The clinical importance of these small increments in haemoglobin levels is questionable. However, one study reported that IV iron resolved anaemia in 62.5% of patients (versus 20% of patients not treated with IV iron; p=0.051).22 Improvements in ferritin levels were significantly greater in patients treated with IV versus oral iron (+165.2 µg/l versus +8.65 µg/l, respectively; p=0.0006), whereas no difference in the change in TSAT was found.47 Vesper et al. reported increases in ferritin of 91.5 µg/l and TSAT of 14.5% after treatment with IV iron (not compared to control).22 IV iron effectively resolved ID, with resolution in 85% of patients (versus 20% not treated with IV iron; p=0.001) and 40% of patients (versus 0% in patients treated with oral iron; p=0.008).22,47 The relative ineffectiveness of oral iron in resolving ID may be attributed to elevated hepcidin levels.23 Furthermore, oral iron may induce oxidative stress in the intestinal mucosa, possibly exposing LVAD patients to an increased risk of GI bleeding, further challenging the justification for the treatment.49 A single study reported greater odds for improvement in New York Heart Association functional class (OR 2.84; 95% CI [1.42–5.68]) after IV iron compared with oral iron, whereas another study did not find a significant difference between treatment groups.47,48 Compared with oral iron, IV iron did not improve 6MWD (+240 ± 220 versus +110 ± 226 m, respectively; p=0.208) at follow-up (within 180 days after implant).22 Because the study was conducted immediately after implantation, the positive effects of IV iron on functional capacity may have been masked by the expected improvement in 6MWD attributable to the LVAD therapy itself.50
Treatment with IV iron was not associated with significant improvements in QoL measures. In the study by Bode et al., five patients had paired Kansas City Cardiomyopathy Questionnaire (KCCQ-12) data, with a median change in the KCCQ-12 score of +9.38 (p=0.075 from baseline), and Vesper et al. reported no difference in the mean change in Minnesota Living With Heart Failure Questionnaire scores between groups.22,47 The sample sizes in these studies were small, and the studies were probably not powered to detect any significant effects of IV iron, making the results inconclusive.
Regarding the safety of IV iron, previous studies have linked IV iron treatment to an increased risk of infection in patients with ID and chronic kidney disease.51 This has led to concerns regarding the treatment of LVAD patients, because device-associated and bloodstream infections are associated with poor outcomes, including increased mortality.42,52,53 Three studies reported safety endpoints after treatment with IV iron, and none reported increased rates of any adverse events, including infection rates, concluding that the treatment is safe in this population.22,47,48 Although sample sizes were small, the results are consistent with the findings in studies of non-LVAD HF patients with ID.2
Importance of Iron Deficiency on Clinical Outcomes
In HF in general, ID is correlated with poorer QoL and worse functional capacity, impairing long-term outcomes.54 However, none of the studies involving LVAD patients reported associations between ID and outcomes in terms of mortality, right ventricle (RV) failure, hospitalisation rates or exercise capacity. Studies are clearly required to address these important topics. Due to the lack of data, we propose two possible mechanisms through which ID may affect adverse outcomes.
First, exercise capacity remains severely impaired following LVAD implantation, affecting mortality, QoL and functional capacity.55–58 In the non-LVAD HF population, ID has been associated with reduced pV̇O2, whereas IV iron repletion has been shown to improve exercise capacity in patients with ID.38,59,60 In LVAD patients, pV̇O2 is associated with haemoglobin levels.61,62 ID is a common cause of anaemia, and treatment with IV iron significantly improved haemoglobin levels while resolving anaemia in most patients.18,22 This may suggest a possible link between exercise intolerance and ID. However, the direct interactions between ID, iron repletion and pV̇O2 have not been studied in LVAD patients.
Second, preserved RV function is crucial during LVAD therapy, because the pump relies on continuous LV filling driven by the RV.15 Early haemodynamic and inflammatory stress often leads to RV dysfunction, and RV failure, which develops in up to 40% of LVAD patients after implant, is a significant mediator of morbidity and mortality during support.63,64 RV failure accounted for 10% of deaths in patients implanted with centrifugal flow LVADs in an analysis of the International Society for Heart and Lung Transplantation Mechanical Assisted Circulatory Support (IMACS) Registry and may have contributed to other causes of death (e.g. multisystem organ failure).65 Several studies in non-LVAD HF patients have linked RV dysfunction to ID; one study found an association between ID and RV dysfunction in patients hospitalised with acute HF, whereas treatment with IV iron has been associated with improved RV function, structure and contractile reserve in non-LVAD HF patients.32,66,67 In LVAD patients, several studies have reported that pre-implantation anaemia and blood transfusions are associated with RV failure.24,68,69 A recent study based on Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) Registry data (n=19,509 LVAD patients), although not statistically significant, found a similar trend between the severity of pre-implant anaemia and rates of postimplant RV failure, and a likely explanation for the association between multitransfusion and RV failure may very well be increased RV afterload rather than reduced contractile force of the RV.62 Please note that, due to the lack of data, the suggested association is extrapolated from studies of non-LVAD HF patients without direct evidence to confirm ID as a causal factor for RV failure in the LVAD population. Still, we believe that this hypothesis warrants further investigation.
Although most of the discussed studies were based on anaemia specifically, ID is a significant cause of anaemia in LVAD patients.18 Furthermore, treating anaemic LVAD patients with blood transfusions leads to unfavourable sensitisation in bridged heart transplantation candidates.70 Previous attempts at improving haemoglobin levels in LVAD patients using erythropoiesis-stimulating agents were deemed unsafe due to higher rates of mortality and suspected pump thromboses.71 Although it is difficult to ascertain the respective influences of anaemia and ID on LVAD patient outcomes based on the available data, haemoglobin levels increase after treatment with IV iron, and thus may limit transfusions in LVAD patients.72
Limitations
Our review of ID in LVAD patients has several inherent limitations. First, it is based on a limited number of studies, exhibiting significant heterogeneity in methodology, research focus and LVAD characteristics. Most of these studies were retrospective and performed at single centres, with small sample sizes (range 7–205 patients). This could potentially limit the generalisability of our findings. Furthermore, there were inconsistencies in the data reported across these studies. Important data points, such as the type of LVAD device, duration of LVAD support and markers of ID, were not uniformly tested and reported. In addition, the iron preparation used was inconsistently reported, which could influence the interpretation of results. The studies were also subject to several biases, including sampling bias due to undisclosed criteria for when ID screening was to be performed and selection bias from non-randomised decisions to treat patients with iron supplementation.
Conclusion
The reported prevalence of ID in HF patients supported with an LVAD ranges from 40 to 82%. Although criteria for diagnosing ID in LVAD patients have not been validated, all studies followed FAIR-HF criteria. TSAT <20% as a lone criterion may increase both the sensitivity and specificity of the screening. Due to the unique inflammatory and circulatory conditions in LVAD patients, oral iron supplementations appear to be ineffective and possibly counterproductive in treating ID. IV iron treatment significantly resolved ID in most patients, leading to increased haemoglobin, ferritin and TSAT levels without being associated with any adverse events.
However, the impact of ID and subsequent iron repletion on survival and exercise capacity after LVAD implantation remains unknown. Although ID has been linked to RV dysfunction, studies of cardiac structure and function in relation to ID have not been conducted in LVAD patients. Because no guideline on diagnosing and treating ID in LVAD patients currently exists, and optimal diagnosing criteria remain unproven, the true prevalence and adverse effects of ID may be severely misestimated. Although no randomised controlled trials of IV iron treatment of ID in patients with LVAD have been conducted, effects similar to those found in the wider HF population may exist.
Next Steps
Several key research topics are relevant to establish the clinical implications of ID in LVAD patients. The relationships between ID and RV dysfunction, mortality, HF hospitalisations, QoL and exercise intolerance should be explored. In addition, mechanistic studies and validation of diagnostic criteria specific to the LVAD population are warranted. Finally, the interaction between anaemia and ID on adverse outcomes should be investigated. Drawing from the insights of this review, we suggest a randomised double-blind placebo-controlled trial examining the effects of IV iron repletion on QoL and pV̇O2, similar to the unblinded FERRIC-HF trial.60 These endpoints are increasingly important, with still improving survival in the LVAD population.
Clinical Perspective
- Iron deficiency remains highly prevalent in HF patients after left ventricular assist device (LVAD) implantation, with prevalence estimates ranging from 40% to 82%.
- The FAIR-HF diagnostic criteria (ferritin <100 µg/l or ferritin 100–299 µg/l and transferrin saturation <20%) are commonly used to diagnose ID; however, transferrin saturation <20% alone may be more accurate in LVAD recipients.
- IV iron repletion improves haemoglobin levels and resolves iron deficiency in most patients; however, effects of iron repletion on mortality, hospitalisation rates, quality of life and functional capacity in LVAD patients require further investigation.
- Future studies are needed to consider the true effects of iron deficiency in the LVAD population.