Heart failure (HF) is a leading cause of morbidity and mortality worldwide.1–3 Despite agreement in treatment recommendations by international HF societies, the use of guideline-directed medical therapy (GDMT) remains suboptimal in chronic HF patients and does not significantly improve after HF hospitalisation.4–7 Hospitalisation for HF signals a poor prognosis with a period of high vulnerability following discharge.8,9 Within 30 days of discharge, approximately 20% of patients are re-hospitalised, and approximately one in 10 patients die.1,3,10,11 Numerous interventions have been studied to improve 30-day outcomes but none has demonstrated significant, reproducible benefits.12–14 In-hospital initiation and titration of GDMT represents a potential solution to improve GDMT usage and subsequently HF outcomes, both in the long term and in the vulnerable post-discharge period. Although large randomised trials have established the safety and efficacy of GDMT initiation and titration in stable, ambulatory patients with HF, newer investigations provide evidence for in-hospital initiation and titration. Here, we review the knowledge gaps in best practices of initiating and up-titrating GDMT, the benefits and risks of in-hospital initiation and post-discharge focused titration of GDMT and the recent literature evaluating these practices, and propose strategies to apply these principles in the care of patients with HF with reduced ejection fraction (HFrEF).
Overview of Medical Therapies for HFrEF
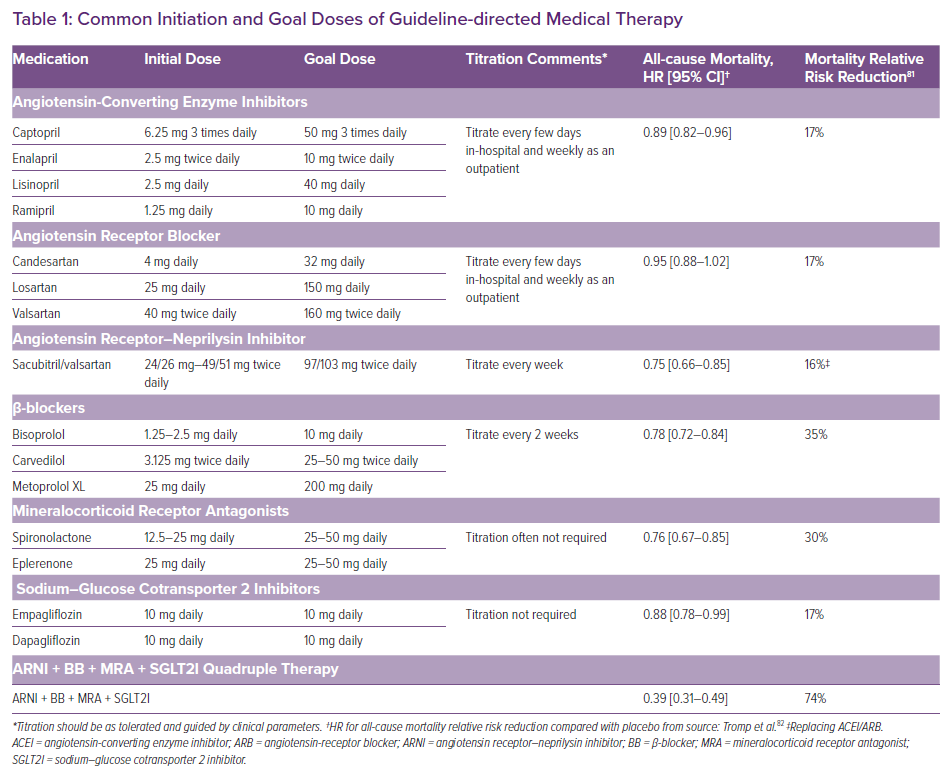
The medical armamentarium for HFrEF includes numerous medications with the potential to decrease mortality and morbidity when initiated and titrated to goal or to maximally tolerated doses in appropriate patients (Table 1). GDMT drug classes recommended for most patients include β-blockers (BBs); angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs) or an angiotensin receptor–neprilysin inhibitor (ARNI), and mineralocorticoid receptor antagonists (MRAs).4,5 Recently, sodium-glucose cotransporter 2 inhibitors (SGLT2Is) were added to GDMT (class I, level of evidence: a) creating four core GDMT classes for HFrEF (left ventricular ejection fraction [LVEF] ≤40%).4,15,16 BB and MRA initiation provides the greatest reduction in mortality risk, followed by replacing ACEI with an ARNI, and then ACEI/ARB and SGLT2I initiation (Table 1).
GDMT recommendations are based upon multiple, randomised clinical trials enrolling ambulatory patients with HFrEF meeting restrictive inclusion and exclusion criteria, and in some instances completion of a tolerability run-in phase prior to randomisation.17–21 Additionally, for BBs, ACEIs/ARBs/ARNIs and MRAs, physicians followed study protocols guiding dose titration to goal doses.
Assessments of GDMT Implementation
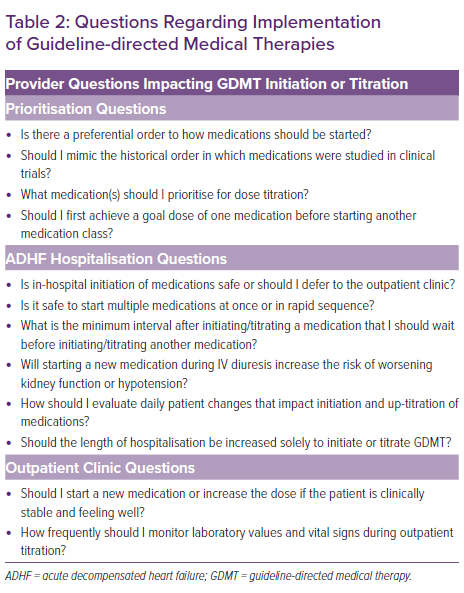
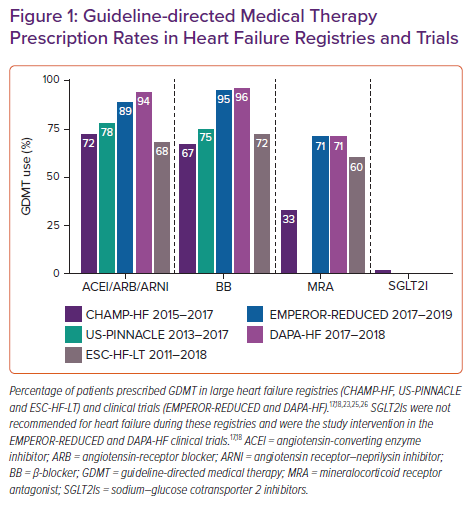
Despite robust scientific evidence confirming the benefits of GDMT, providers are left with valid questions regarding GDMT implementation, many of which do not yet have evidence-based answers (Table 2). Consequently, an alarming percentage of patients who would benefit are not prescribed GDMT. National registries and clinical trials often provide unique insights into gaps in GDMT implementation and illustrate a sizable difference in GDMT prescription rates between clinical trial and real-world cohorts (Figure 1). Change the Management of Patients with Heart Failure (CHAMP-HF) is one such national registry of HFrEF patients receiving ≥1 oral HF medication.22 In a 2018 analysis of the CHAMP-HF registry, Greene et al. showed that in this cohort (mean LVEF, 29 ± 8%) many patients were not prescribed ACEI/ARB/ARNI (27%), BB (33%) or MRA (67%) therapies despite the lack of contraindication to these medications.23 Lower percentages of patients were receiving each of these three classes of medication at recommended goal doses (17% ACEI/ARB, 14% ARNI, 28% BB and 77% MRA). Only 1% of patients simultaneously received all three classes of medication at goal doses. Furthermore, at 12-month follow-up very few patients had medication initiation or up-titration (7% ACEI/ARB, 10% ARNI, 10% BB and 6% MRA).24
Similar rates of GDMT implementation have been reported from the US PINNACLE registry involving more than 6 million patients. As of 2017, 25.4% of HFrEF patients were still not prescribed a BB, and 27.2% were not prescribed an ACEI/ARB/ARNI.25 Patterns of GDMT under-utilisation are similar in the European Society of Cardiology Heart Failure Long-Term (ESC-HF-LT) registry, indicating that this issue persists across different healthcare and reimbursement systems.26
Since the publication of these real-world registries, SGLT2Is have been recommended as an additional GDMT for HFrEF.4,15 Only 2% of patients in CHAMP-HF were treated with SGLT2Is but it is important to note that this registry was enrolled from 2015 to 2017 prior to any guideline recommendation. Although an increase in usage could be anticipated, current assessments of prescribing patterns for SGLT2I indicate a continued pattern of under-utilisation in patients with established benefits.27 Overall, the rates of implementation of GDMT in real-world populations remain inadequate, with up to one-third of eligible patients failing to receive therapy. Furthermore, the implementation of novel therapies, such as ARNIs and SGLT2Is, is also low, despite randomised controlled trials establishing their benefit.
In-hospital GDMT Initiation and Titration
In-hospital GDMT initiation and titration during acute decompensated HF (ADHF) is one potential way to improve GDMT implementation. Studies have shown that hospitalisation with ADHF is strongly associated with an increased risk of mortality and 30-day rehospitalisation.3,10,11 However, GDMT prescription rates change minimally after ADHF hospitalisation. An analysis of 115,220 ADHF hospitalisations from 276 hospitals participating in the Get With The Guidelines HF Registry from 2005 to 2009 showed that the rate of GDMT addition from admission to discharge increased from 10 to 20% for MRA, 45 to 72% for ACEI/ARB and 38 to 84% for BB.6 This suggests that there is substantial room for improvement in using the hospital episode to initiate GDMT.
Although poor GDMT implementation is likely to be multifactorial (i.e. socioeconomic factors, patient-related factors, provider assessment of risk, medication intolerance), clinical inertia, defined as a hesitancy to make medication changes, appears to be at least one major factor.28,29 A national-level analysis comparing GDMT regimens 3 months before with those 3 months after a worsening HF event in patients with HFrEF reported similar rates of patients on one class of GDMT (29–29%), two GDMT classes (39–42%) and three GDMT classes (14–17%) following the worsening HF event, with these low rates persisting 1 year later.30 In addition to reinforcing previous reports, that study provided a unique insight into the potential barriers to GDMT implementation. By studying younger patients with commercial insurance, it was found that low GDMT implementation is unlikely to be predominantly driven by medication intolerance due to advanced age, comorbidities or financial barriers.30 Similarly, alerting providers to a patient’s prognosis also did not improve GDMT prescription rates in a recent randomised trial, diminishing the likelihood that provider misunderstanding of prognostic risk is responsible for poor GDMT implementation.31,32
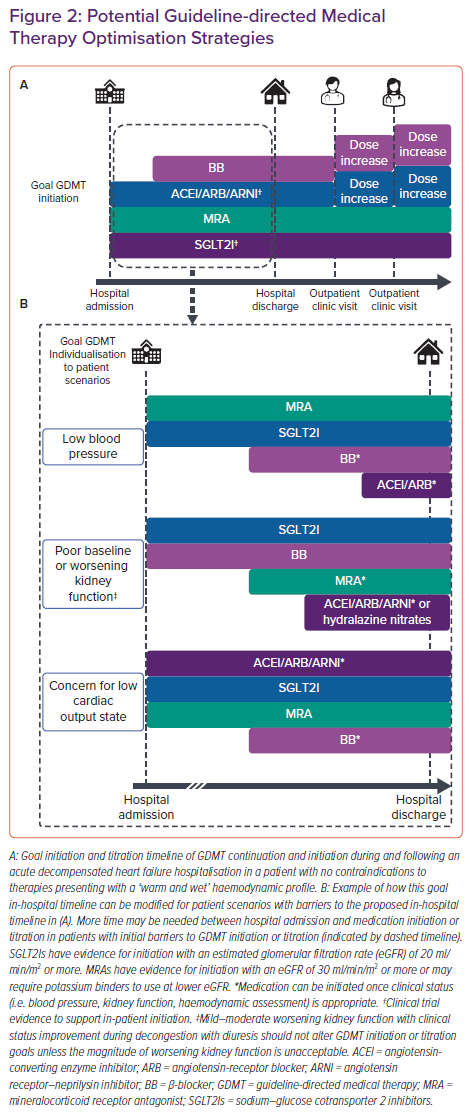
GDMT initiation and titration during ADHF hospitalisation is an emerging strategy to increase both acute GDMT implementation and chronic adherence. HF guidelines, consensus statements and clinical experts now advocate for in-hospital, rapid sequence initiation of core GDMT including BBs, ACEIs/ARBs/ARNIs, MRAs, and SGLT2Is.4,7,15,33–35 A goal for patients with HFrEF hospitalised with ADHF is to achieve initiation or continuation of all four classes of GDMT by hospital discharge in appropriate patients (Figure 2A). However, achievement of this goal will require significant changes to the conventional approach to GDMT optimisation. Historically, providers initiated GDMT classes in the order in which they were studied in clinical trials: ACEI or ARB followed by BB, then MRA initiation, and finally SGLT2I initiation. This process could further be lengthened by transitioning the ACEI/ARB to an ARNI in line with clinical trial evidence.19
Strategies reordering this historical sequence and timeline have been proposed, minimising the time to quadruple GDMT achievement.34,36 The theoretical reasons for these approaches are multifaceted.34,36 First, the beneficial effects of each GDMT class are independent of the other classes. Second, low doses of ACEI/ARB reduce the mortality and morbidity risk, and the additive benefit of adding another GDMT class is greater than the benefit of titrating to the goal dose.18,20,37,38 Third, the benefits of GDMT can be seen as early as 30 days after initiation, minimising delays in benefits during a high-risk period.36,39–41 Finally, prescription at the time of hospital discharge increases the likelihood of chronic medication adherence and GDMT prescription.42,43
Efficacy and Safety of In-hospital GDMT Initiation
No prospective study has evaluated the strategy of simultaneous or rapid sequence GDMT class initiation in ADHF patients. Presently, randomised trials and registry data are limited to evaluating the addition of a single GDMT class during ADHF hospitalisation to the background therapies.
Sodium–Glucose Cotransporter 2 Inhibitors
SGLT2Is demonstrate a clear benefit with minimal risk during in-hospital initiation. The efficacy and safety of in-hospital initiation of empagliflozin during ADHF was evaluated in a multicentre randomised controlled trial (EMPULSE).44 Empagliflozin 10 mg once daily or placebo was started between days 1 and 5 of ADHF hospitalisation in 530 patients. Approximately 45% of patient had diabetes and the majority had a reduced ejection fraction (~65%). The primary composite outcome of all-cause death, number of worsening HF events, and change in Kansas City Cardiomyopathy Questionnaire symptom score was assessed at 90 days. Patients in the empagliflozin group were 36% more likely to achieve clinical benefit (win ratio 1.36; 95% CI [1.09–1.68]; p=0.0054), and this benefit was consistent across all components of the primary outcome (all-cause mortality, 4.2% with empagliflozin versus 8.3% with placebo).45 Fewer patients had a serious adverse event with empagliflozin than placebo (32.3% versus 43.6%, respectively). Providers may have concerns for specific adverse events with SGLT2Is during ADHF hospitalisation. Empagliflozin (versus placebo, respectively) did not increase the risk of hypovolemic hypotension (empagliflozin 10.4% versus placebo 10.2%), acute kidney injury (7.7% versus 12.1%), hypoglycaemia (1.9% versus 1.5%), ketoacidosis (no cases), or urinary tract infection (4.2% versus 6.4%). Similar benefits and risks were found with sotagliflozin in the SOLOIST-WHF trial, although sotagliflozin was initiated on or within 3 days of hospital discharge.46
ACEI/ARB/ARNI
Although randomised trials have not evaluated the acute outcomes of in-hospital initiation of ACEI or ARB versus placebo, the CONSENSUS trial randomised 253 patients hospitalised with ADHF to enalapril 5 mg twice daily or placebo and evaluated the outcomes at 6 months and 1 year.47 The timing of ACEI initiation during the hospital episode was not specified. Enalapril titrated to a mean daily dose of 18.4 mg significantly improved all-cause mortality by 40% and 31% at 6 months and 1 year, respectively, without increasing the rates of worsening kidney function or hyperkalaemia. Hypotensive events occurred more frequently with enalapril (7 events versus 0 events with placebo), prompting a change in the starting dose to 2.5 mg daily in patients at risk of hypotension. Although not evaluated statistically, the mortality benefit appeared to accrue almost immediately following randomisation.
In-hospital initiation of sacubitril–valsartan or enalapril was evaluated in the multicentre, double-blinded, randomised PIONEER trial.48 Patients were eligible 24 hours after presentation once they were medically stable, defined as no IV inotropic medication in 24 hours and no IV vasodilator medication, IV diuretic dose escalation, or systolic blood pressures below 100 mmHg for the previous 6 hours. Patients were enrolled a median of 68 hours (IQR 48–98 hours) after presentation to the hospital and the majority were not yet decongested. Therapy was initiated as sacubitril/valsartan 24/26 mg or enalapril 2.5 mg twice daily unless the systolic blood pressure exceeded 120 mmHg, in which case sacubitril/valsartan 49/51 mg or enalapril 5 mg twice daily was initiated. Doses were titrated at 1, 2, 4 and 6 weeks as tolerated to goal doses. At 8 weeks, ARNI therapy significantly decreased the natriuretic peptide concentration, and also reduced the number of rehospitalisations for HF compared with enalapril (8.0% versus 13.8%; HR 0.56; 95% CI [0.37–0.84]). Rates of hyperkalaemia and worsening renal function were not different between the groups and were consistent with other ADHF trials and registries.
However, this trial had a high drop-out rate in both treatment arms, which resulted in a missing natriuretic peptide concentration in 15% of the population and limited the comparisons with other trials. The importance of this high drop-out rate in evaluating the early benefits and tolerance of ARNI is accentuated by the LIFE trial that randomised 335 patients with advanced HFrEF (i.e. recent IV inotrope use, ≥1 ADHF hospitalisation in the past 6 months, LVEF <25%, peak VO2 <55% predicted, or 6-minute walk distance <300 m) and recent New York Heart Association class IV symptoms to sacubitril–valsartan or valsartan alone.49 Unlike the PIONEER trial, the LIFE trial did not find a reduction in natriuretic peptides, and the reasons for the contrasting results may include differences in patient populations, disease severity, and the comparison treatment arm, among other variables. Notably 18% of subjects were not able to tolerate sacubitril–valsartan 24/26 mg twice daily during the short run-in period, and 29% of patients discontinued sacubitril–valsartan during the 24-week trial. Therefore, appropriate patient selection is important to ensure ARNI tolerance during initiation and titration.
Registry data of ACEI/ARB use during ADHF hospitalisation reinforce these clinical trial data. Data from more than 16,000 patients in the Get With The Guidelines HF Registry were combined with Medicare claims data to compare ACEI/ARB initiation, continuation and non-initiation during ADHF hospitalisation.50 After multivariate adjustment, similar 30-day mortality rates were found between patients starting ACEI/ARB and those continuing home ACEI/ARB (4.1% versus 3.5%), but a higher 30-day mortality rate (8.8%; HR 1.92; 95% CI [1.32–2.81]) was found for those for whom ACEI/ARB therapy was not started in hospital.50 This mortality benefit of in-hospital ACEI/ARB initiation has been substantiated in other observational cohorts.51
β-blockers
Evidence for BB in-hospital initiation is limited compared with other GDMT classes but favourable. An open-label, randomised trial compared in-hospital with post-discharge carvedilol initiation in 363 patients with HFrEF hospitalised with ADHF.43 In-hospital initiation significantly increased the use of BBs at 60 days after discharge without increases in adverse events, worsening HF, rehospitalisation or death, although the trial was not powered for assessment of clinical outcomes. In the OPTIMIZE-HF registry, 935 patients were deemed appropriate candidates for in-hospital BB initiation prior to discharge.42 In-hospital BB initiation was associated with a lower 60-day mortality (HR 0.41; 95% CI [0.22–0.78]; p=0.006) and combined death–rehospitalisation rate (HR 0.61; 95% CI [0.44–0.83]; p=0.002) compared with those for eligible patients not starting BBs.
Subsequent analysis of 3,001 patients with ADHF in the OPTIMIZE-HF registry with in-hospital BB initiation found that these early benefits were still present at 1 year in a larger cohort.52 Collectively, evidence supports the efficacy and safety of in-hospital BB initiation in appropriate candidates who have been adequately treated and are no longer in a decompensated state.
Mineralocorticoid Receptor Antagonists
Assessment of the acute benefits of in-hospital MRA initiation is limited to observational analyses with less robust data than other GDMT classes. In a retrospective propensity score-matched analysis of the ALARM-HF registry, in-hospital MRA use was associated with reduced all-cause mortality during hospitalisation.53 In-hospital MRA initiation significantly reduced 30-day death and HF rehospitalisation in a retrospective analysis of the COACH trial.54 In contrast, no benefit in 30-day death or hospitalisation was found with in-hospital MRA initiation using Medicare claims data.55 The reasons for the discrepancy between randomised clinical trials consistently demonstrating benefit with MRA initiation in HFrEF and the inconsistent benefit observed in these analyses is likely to be multifactorial and influenced by confounding bias from patient selection and clinical trial exclusion criteria rather than being due to the timing of initiation, given that the EPHESUS trial found 30-day benefits with MRA therapy in HFrEF with initiation at a mean of 7 days after acute MI.56
Although efficacy is not conclusively demonstrated in registry data, the safety of in-hospital MRA efficacy is bolstered by randomised trial evidence. The ATHENA trial randomised patients to in-hospital initiation of spironolactone at 25 or 100 mg daily doses for 4 days during ADHF hospitalisation.57 Even at doses 2–3-fold higher than recommended chronic spironolactone doses, there was no evidence of increased adverse event risk, such as hyperkalaemia, hypotension or kidney dysfunction, in patients with a glomerular filtration rate (GFR) ≥30 ml/min/1.73 m2, serum potassium ≤5 mEq/l and systolic blood pressure >90 mmHg at baseline.
Adapting GDMT Initiation and Titration to Clinical Scenarios
Although in-hospital GDMT of a single medication class appears safe and efficacious, clinicians may have questions regarding the application of these data to rapid sequence optimisation or the spectrum of ADHF phenotypes (Table 2). HF guidelines recommend initiation or up-titration of GDMT during ADHF hospitalisation but lack information on the order or method of up-titration.4,5 Established initiation and goal doses are listed in Table 1 along with in-hospital and outpatient titration considerations. In patients presenting with a ‘warm and wet’ haemodynamic profile without contradictions to GDMT, home GDMT should be continued and low doses of new GDMT can be initiated early in hospitalisation, with the possible exception of BB initiation (Figure 2A). BB therapy can be initiated once the patient is clinically improving and prior to hospital discharge.
Patients presenting with complications may require reordering and delay of some GDMT classes until later in the hospital episode. ADHF admissions complicated by cardiogenic shock, acute coronary syndrome or worsening kidney function are not uncommon in registries, and patients with these scenarios were either excluded from inpatient initiation trials or they could not be enrolled until the complications were resolved.26,44,48,58
Recently, expert statements have suggested that titration and prioritisation strategies be based on patient phenotype, considering heart rate, arrhythmia, blood pressure and kidney function.59,60 However, the complexity of tailoring GDMT to phenotype is high and not guided by evidence. Phenotypes can change significantly during ADHF hospitalisation and numerous permutations of GDMT prioritisation exist. Figure 2B provides examples of commonly encountered clinical scenarios that may affect the order of GDMT initiation or titration.
The example of a patient with low blood pressure is both illustrative of how GDMT can be tailored to a phenotype and indicative of the complexity involved in individualisation even within a phenotype. A patient with low blood pressure potentially limiting initiation of ACEI/ARB/ARNI or BB presently could be initiated on MRA and/or SGLT2I therapy first, given that these therapies have no to minimal effect on blood pressure.61,62 However, patients with low blood pressure and an elevated systemic vascular resistance on haemodynamic monitoring may benefit from prioritisation of vasodilators without hypotension. Thus, the heterogenicity of data available and the haemodynamic statuses within phenotypes limits definitive statements on GDMT prioritisation at even the phenotype level.
Acknowledging the limitations of phenotype-guided GDMT initiation and titration, some guidance for common scenarios can be extracted from HF clinical trials. In chronic kidney disease, GDMT has established benefits down to an estimated GFR (eGFR) of 15 ml/min/1.73 m2.63 Inpatient ARNI and SGLT2I initiation required an eGFR >30 and >20 ml/min/1.73 m2, respectively.45,48 MRAs are recommended in patients with an eGFR >30 ml/min/1.73 m2 with close potassium monitoring.5 Worsening kidney function after initiation, especially if during IV diuresis, should be evaluated in the context of overall clinical status and should not automatically necessitate GDMT discontinuation.64 Both ARNI and SGLT2I were initiated during ADHF hospitalisation in patients with a systolic blood pressure >100 mmHg, while carvedilol was initiated in patients with severe HFrEF and a systolic blood pressure >85 mmHg.45,48,65 However, patients with low blood pressures receiving ARNI, ACEI or carvedilol were more likely to stop therapy or have symptomatic hypotension than patients with higher blood pressures at initiation.66,67 MRA trials did not have a blood pressure exclusion criterion and were well tolerated in patients with a systolic blood pressure <105 mmHg.62 Finally, GDMT initiation during ADHF hospitalisation should be delayed until the patient is haemodynamically stable. Criteria used in the in-hospital initiation trials included being off IV inotropes for 24 hours, having stable blood pressure for 6 hours, and at least 6 hours without IV vasodilators.44,48
Minimal evidence exists to guide titration prioritisation. CIBIS III demonstrated no significant difference in mortality or HF hospitalisation between a strategy prioritising ACEI versus that prioritising BB, albeit in patients with chronic HF.68 However, given the lower magnitude of clinical benefit from dose titration in ACEI/ARB compared with additional GDMT class initiation, initiation of all four GDMT classes should be prioritised over dose titration of a single GDMT class.37,38,69 Titration to goal doses is important and should subsequently be pursued in patients tolerating GDMT.
Medical therapies for HF outside of the core four GDMT classes can also benefit patients with HFrEF and may be considered for in-hospital initiation. Patients admitted with ADHF should be screened for iron deficiency. In patients with iron deficiency, initiation of IV iron repletion prior to hospital discharge and completion of repletion in the post-discharge period did not increase adverse events and demonstrated a reduction in the risk of future ADHF hospitalisation.70
Additional interventions to improve outcomes in selected populations include hydralazine and isosorbide dinitrate, ivabradine, digoxin, and vericiguat. In-hospital initiation data for these therapies, however, are limited. In African–American patients the addition of hydralazine and isosorbide dinitrate to the above-mentioned GDMT has benefit and can be initiated in hospital in patients on goal doses of core GDMT. Hydralazine and isosorbide dinitrate may also be considered in patients unable to tolerate ACEI/ARB/ARNI.4
Ivabradine should be considered for persistently symptomatic patients with a sinus rhythm heart rate ≥70 BPM despite maximally tolerated BB and GDMT, although candidates should be evaluated once compensated and stable.15 Digoxin improved the composite endpoint of HF mortality or hospitalisation in high-risk patients and can be considered for persistently symptomatic patients despite GDMT or inability to tolerate GDMT due to hypotension.71 In the VICTORIA trial, vericiguat was initiated at 2.5 mg daily and titrated every 2 weeks to a goal dose of 10 mg daily in patients with recent worsening HF.72 Vericiguat may be considered for persistently symptomatic patients despite core GDMT therapies.4
Continuing GDMT Optimisation in the Post-discharge Phase
The morbidity and mortality benefits of GDMT demonstrated in pivotal clinical trials of chronic HFrEF are derived over months to years of pharmacotherapy at goal or maximally tolerated doses. Presently, cost-effectiveness studies comparing the prolongation of hospitalisation to optimise GDMT with outpatient GDMT titration are lacking. Although ADHF hospitalisation provides a unique opportunity to closely monitor the initiation and titration of GDMT, the maximum long-term benefits can only be realised through outpatient adherence and subsequent titration to goal doses as appropriate.
Successful hospital discharge involves appropriate patient education, medication access, and communication between care teams. Throughout the hospitalisation the patient should be engaged as an active stakeholder in their care, given that patient education during the hospitalisation may improve adherence to pharmacotherapy at hospital discharge.73 Additionally, social determinants of health compound HF complexity, particularly at the point of hospital discharge.74 Upfront costs deter vulnerable patients from chronic medication adherence.74 Systematic interventions providing short-term medication access have largely been successful in promoting medication adherence without affecting clinical outcomes; a multidisciplinary approach involving pharmacists, case managers and social workers may improve sustainable medication access.74 Finally, effective communication between inpatient and outpatient treatment teams is a core component of successful GDMT optimisation across episodes of care. A standardised focused discharge hand-off including the discharge GDMT regimen and plans for further optimisation may facilitate this transition.33
After hospital discharge, minimal GDMT dose titration occurs.24,30 Clinical inertia is often driven by a fear of causing adverse effects in apparently clinically stable patients; however, failure to further initiate and titrate GDMT to goal doses may worsen the quality of life, increase HF hospitalisations and decrease survival.28 Several mechanisms to promote outpatient optimisation of GDMT have been investigated, including multidisciplinary titration clinics, electronic health records (EHR) and artificial intelligence. HF guidelines recommend (class 1) multidisciplinary management of patients with HF.4,5 Multidisciplinary GDMT titration clinics have emerged as an effective mechanism to optimise GDMT and improve clinical outcomes.75 Multidisciplinary GDMT titration clinics, often including nurses and pharmacists, optimise GDMT using treatment and monitoring algorithms under the supervision of a cardiologist.75 EHRs are powerful tools to both identify patients with HF across a medical system and promote GDMT optimisation.76–79 The EHR alerts alone, however appear to be of limited value unless combined with interventions to also improve HF outcomes.31,32,79 Artificial intelligence has the capability to improve therapeutic recommendations although a positive impact on clinical outcomes is yet to be reliably demonstrated.80
Conclusion
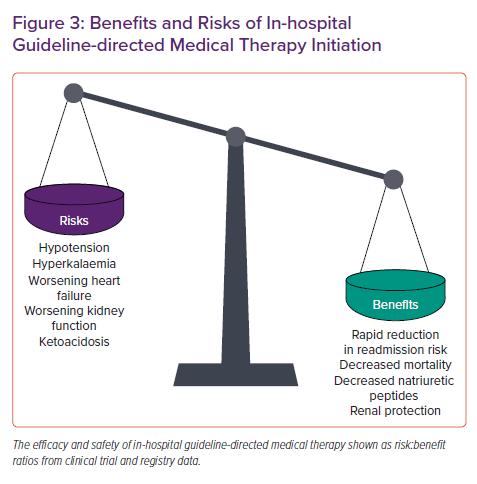
In summary, substantial gaps exist in optimal GDMT for HF. In-hospital GDMT initiation and titration has a rapidly expanding body of evidence to support its efficacy and safety (Figure 3), but many implementation questions (Table2) remain unanswered. Clinicians should use the ADHF hospital episode to create a GDMT optimisation plan spanning episodes of care, optimise GDMT in appropriate patients, overcome barriers to chronic medication access, and communicate these plans to the outpatient care team. Future studies can guide the application of in-hospital GDMT initiation and titration to patient-specific phenotypes and clinical scenarios.