The cut-off values for “normal” ejection fraction (EF) are poorly defined. The EchoNoRMAL study suggested a lower boundary of 49–57 %.1 The American Society of Echocardiography and European Association of Cardiovascular Imaging consider a normal EF and normal range (±2 SD) as 62 % (52–72 %) in men and 64 % (54–74 %) in women.2 By these criteria, an EF of 40–49 % would not be considered normal.
However, there is considerable uncertainty and even controversy around the newly defined heart failure (HF) category of “HF with mid-range ejection fraction” (HFmrEF; EF 40–49 %). The 2016 European Society of Cardiology HF guidelines introduced this term for HF with EF in the middle range of 40–49 %, which is between HF with reduced EF (HFrEF; <40 %) and preserved EF (HFpEF; ≥50 %) EF.3,4 While the purpose of creating this category was to identify an area in need of further research, it has led to some confusion regarding how to classify and, more importantly, how to treat patients with HFmrEF.
The Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Programme studied patients with symptomatic heart failure across the entire spectrum of EF. In CHARM-Preserved, which enrolled patients with LVEF >40 %, candesartan did not significantly reduce cardiovascular death or HF hospitalisation (unadjusted HR 0.89 [95 % CI 0.77–1.03], p=0.118; covariate adjusted 0.86 [0.74–1.0], p=0.051). However, it was effective in HFrEF and, in CHARM-Overall, there was no heterogeneity with respect to EF (p=0.33). Recently, we specifically studied HFmrEF in CHARM and tested the hypothesis that candesartan improves outcomes in HFmrEF.5
HFmrEF Characteristics: Similar to HFrEF
HFmrEF is often referred to as an “intermediate” phenotype that may represent a “transition phase”, but several reports over the past year suggest that this is overly simplistic6–11.
HFmrEF made up about 10 % of incident HF in a US community-based study.12 In prevalent HF, it represents 24 % in the European Society of Cardiology Heart Failure Long-Term Registry,8 21 % of the Swedish HF Registry (SwedeHF),11 and 13 % of a multi-ethnic Singapore and New Zealand cohort.13
While many characteristics in HFmrEF are intermediate between HFrEF and HFpEF, many others – especially the higher prevalence of ischaemic heart disease – suggest that HFmrEF is distinctly more similar to HFrEF.6
In CHARM, HFmrEF accounted for 17 % of patients. It was indeed intermediate between HFrEF and HFpEF with regard to history of hypertension, New York Heart Association (NYHA) class, and BMI. However, HFmrEF appeared similar to HFrEF regarding the most important characteristics, e.g. lower age, male sex predominance, lower systolic blood pressure, less AF, and more ischaemic heart disease and a history of MI, consistent with other emerging analyses.14
HFmrEF Outcomes: More Similar to HFpEF
In CHARM, over a mean follow-up of 2.9 years overall, there were: 15.9, 8.5, and 8.9 primary events (cardiovascular deaths or first HF hospitalisations) per 100 patient-years in HFrEF, HFmrEF and HFpEF respectively; and 20.0, 10.8, and 11.1 recurrent HF hospitalisations per 100 patient-years respectively. The incidence rates for first HF hospitalisation, cardiovascular (CV) death and all-cause death were comparable in HFmrEF and HFpEF and lower in HFmrEF and HFpEF than in those with HFrEF.
While the characteristics of HFmrEF are distinctly more similar to HFrEF, the syndrome appears milder in HFmrEF, so the CV risk appears lower.
Candesartan Appears Effective in HFmrEF
In CHARM, the incidence rates for the primary outcome for candesartan versus placebo were 14.4 versus 17.5 per 100 patient-years (HR [95 % CI] 0.82 [0.75–0.91], p<0.001) in HFrEF; 7.4 versus 9.7 per 100 patient-years (0.76 [0.61–0.96] p=0.02) in HFmrEF; and 8.6 versus 9.1 per 100 patient-years (0.95 [0.79–1.14] p=0.57) in HFpEF. For recurrent HF hospitalisation, the incidence rate ratios were 0.68 (0.58–0.80), p<0.001; 0.48 (0.33–0.70), p<0.001; and 0.78 (0.59–1.03), p=0.08, respectively.
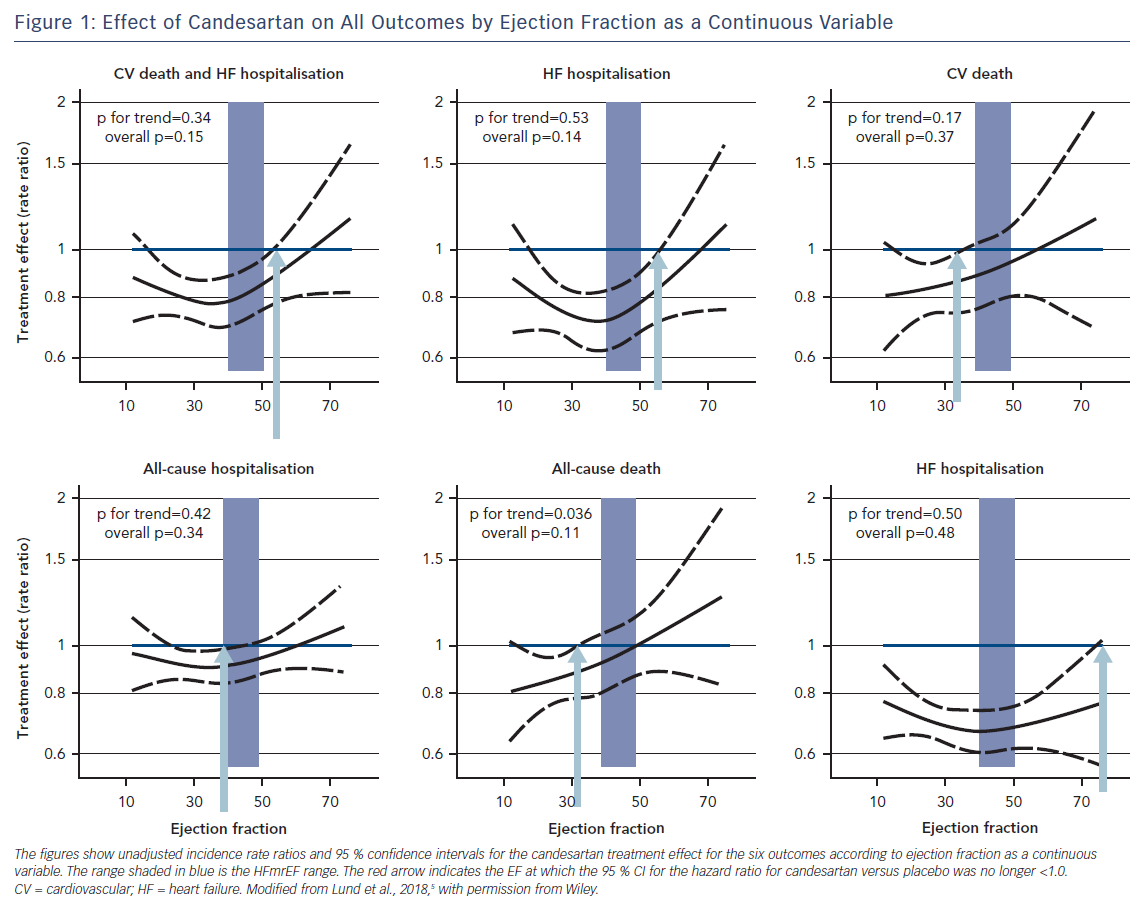
Figure 1 shows unadjusted treatment effects for each outcome according to continuous EF (spline). The hazard ratios and upper 95 % CIs were all below 1.0, indicating benefit with candesartan, up to and beyond EF ~50 % for the primary composite and first HF hospitalisation outcomes, and up to EF ~60 % for the recurrent HF hospitalisations outcome.
Candesartan reduced CV death, all-cause death and all-cause hospitalisation only at the lower end of the EF spectrum. The potential efficacy of HFrEF therapy also in HFmrEF has been hinted at in observational studies,15,16 in the TOPCAT trial with spironolactone,17 as well as in a meta-analysis of 11 randomised trials with beta-blockers.18
Taken together, these data provide a rationale for future studies of generic, inexpensive treatments in HFmrEF, something that can be done at low cost and high efficiency in pragmatic trial settings.19
However, the resemblance between HFmrEF and HFrEF and the benefits suggested in the post-hoc analyses of CHARM, TOPCAT and the beta-blocker meta-analysis may make clinicians reluctant to randomise patients in the HFmrEF range, and the variability of EF measurements may make it difficult to identify patients with HFmrEF reliably.
Conclusion
The recent analysis from CHARM suggests that:
- HFmrEF resembles HFrEF regarding most clinical characteristics, in particular a history of myocardial infarction.
- HFmrEF resembles HFpEF with respect to a lower risk of HF and CV events, suggesting HFmrEF is a milder syndrome than HFrEF.
- Candesartan may reduce CV and HF events in HFmrEF to the same extent as in HFrEF.
EF may change over time and there is inherent variability in EF measurements, which makes identification of patients with HFmrEF difficult; nonetheless, this condition is not infrequently encountered and it needs to be addressed.
The treatment effect finding in CHARM should be interpreted with caution because this was a post-hoc analysis; nonetheless, it suggests that interventions known to be effective in HFrEF have potential and should be explored also in HFmrEF.







