Sodium–glucose cotransporter 2 inhibitors (SGLT-2i) have become a cornerstone in the treatment of heart failure (HF) irrespective of aetiology and ejection fraction.1 The DAPA-HF trial was the first trial to investigate the effects of SGLT-2i in patients with heart failure with reduced ejection fraction (HFrEF). The trial demonstrated a reduced occurrence of worsening HF, hospitalisation for HF and death from cardiovascular causes.2 In addition, the study showed that dapagliflozin was equally effective in non-diabetic heart failure patients indicating a mechanism of action that was independent of its glucose-lowering effect. The EMPEROR-Reduced trial corroborated these findings in HFrEF patients with more severe systolic dysfunction.3
Whereas significant progress in the treatment of HFrEF patients was made prior to the discovery of SGLT-2i treatment, the development of effective treatments in HF with preserved LVEF (HFpEF) has been limited.4 The EMPEROR-Preserved trial observed a decrease in the composite endpoint of hospitalisation for HF or cardiovascular disease.4 The DELIVER trial similarly investigated the effect of SGLT-2i on the composite endpoint of worsening HF or cardiovascular death in patients with HFpEF and found a significant reduction primarily driven by a decrease in worsening HF.5
The pharmacological action of SGLT-2i is primarily an increase in the urinary excretion of glucose, sodium and water by inhibition of the sodium glucose co-transporter mediated reabsorption of glucose in the proximal tubules. The underlying mechanisms of the observed clinical effects of the SGLT-2i drug class in patients with clinical HF and risk factors for the development of HF have been subject to intense debate and are likely to be multifactorial. Suggested cardiac factors include reduced aortic stiffness, reduced blood pressure, reduced cardiac fibrosis, improved mitochondrial function, increased adenosine triphosphate production and improved ventricular contractile function.6–8 Elevated cardiac filling pressures are a hallmark of HF and are directly linked to symptoms and outcome.9 Increased excretion of sodium and water could potentially lead to lowering of filling pressures and, consequently, improvement of central haemodynamics that could be of significant importance in the mechanisms underlying the clinical benefit seen with SGLT-2i in HF. However, a clear association between any of these factors and the evident clinical effect of SGLT-2i remains to be validated. The aim of this narrative review was to describe and synthesise the available literature describing haemodynamic effects of SGLT-2i in HF. This is the first review to exclusively focus on the haemodynamic effects in HF patients and whether they seem to explain the improved patient outcomes.
Material and Methods
A search was performed in PubMed up to 18 September 2023, and ‘all fields’ were chosen for the search to increase the likelihood of encountering relevant trials containing data on cardiac function and remodelling. Using the primary aspects, a search string was made, which can be found in Supplementary Table 1.
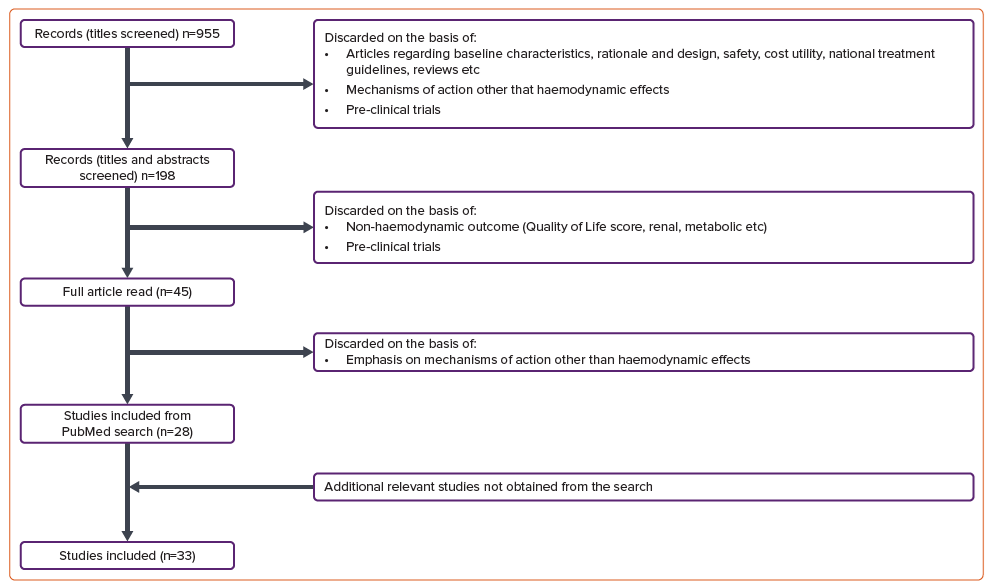
We included studies with haemodynamic investigations containing all three primary criteria in a matter of association, so that the change in haemodynamic changes from treatment with SGLT-2i could potentially be put in the context of explaining the improvement in mortality and hospitalisation from HF. Papers were excluded if they had a lack of relevance in terms of a mechanism-of-action (MoA) approach, such as articles regarding design and rationale, baseline characteristics, safety and cost-effectiveness of the treatment. Trials with an MoA approach but without cardiac haemodynamic measurements, such as articles regarding shifts in cardiac metabolism, renal outcomes, plasma volume regulation and so on were not included. In addition, pre-clinical trials were excluded. This screening of articles from the search is visualised in Figure 1.
Results
Effect on Pulmonary Artery Pressure
Seven studies have investigated the effect of SGLT-2i on pulmonary artery pressure (PAP) in HFrEF patients (Table 1). Although the studies differ with respect to patient selection criteria, endpoint definition and methodology, a clear trend towards a rapid reduction in pulmonary artery pressures is observed across the studies. The EMBRACE-HF trial investigated the effects of SGLT-2i treatment on PAP and observed a reduction after 1 week of treatment, which continued throughout the treatment period of 12 weeks.10 Kirschbaum et al. also observed a decrease in PAP (in both systolic, diastolic and mean PAP) following 10 weeks of treatment.11 The decrease was evident after 3 weeks. Mullens et al. investigated the short-term effects and similarly observed a decrease in mean PAP, evident after just 2 days of treatment.12 It was however a small study of nine HFrEF patients measuring the decrease in PAP following 1 week of treatment with an area under the curve methodology averaging the results from the week. These results support the fast-onset effect. In the GLISCAR study, systolic PAP remained decreased following 6 months of treatment, suggesting a sustained long-term effect.13 Hwang et al. used echocardiography to estimate systolic PAP and observed a significant decrease for their patients with HFrEF.14 Camci et al. observed a decrease in mean PAP. Omar et al. observed no decrease in diastolic or mean PAP at rest but did observe a reduction in diastolic PAP at peak exercise.15
The results of studies in patients with HFpEF on the effect of SGLT-2i on PAP are ambiguous. The EMBRACE-HF trial found the reductions in PAP to be consistent in both HFrEF and HFpEF patients and the CAMEO-DAPA trial observed reductions in mean PAP at exercise but not at rest.16 In contrast, Hwang et al. did not observe a decrease in PA pressure in their group of HFpEF patients.14
Effect on Left Ventricular Filling Pressure or Pulmonary Artery Wedge Pressure
Omar et al. examined the impact of empagliflozin by right heart catheterisation and demonstrated a decrease in pulmonary capillary wedge pressure (PCWP).15 This was corroborated non-invasively by the GLISCAR study and by Hwang et al. who demonstrated a significant reduction in E/e’ ratio, but this was not confirmed in the CANA-HF trial, DAPA-VO2 trial or the CANDLE trial where a reduction in E/e’ was not observed (Table 1).13,14,17–19
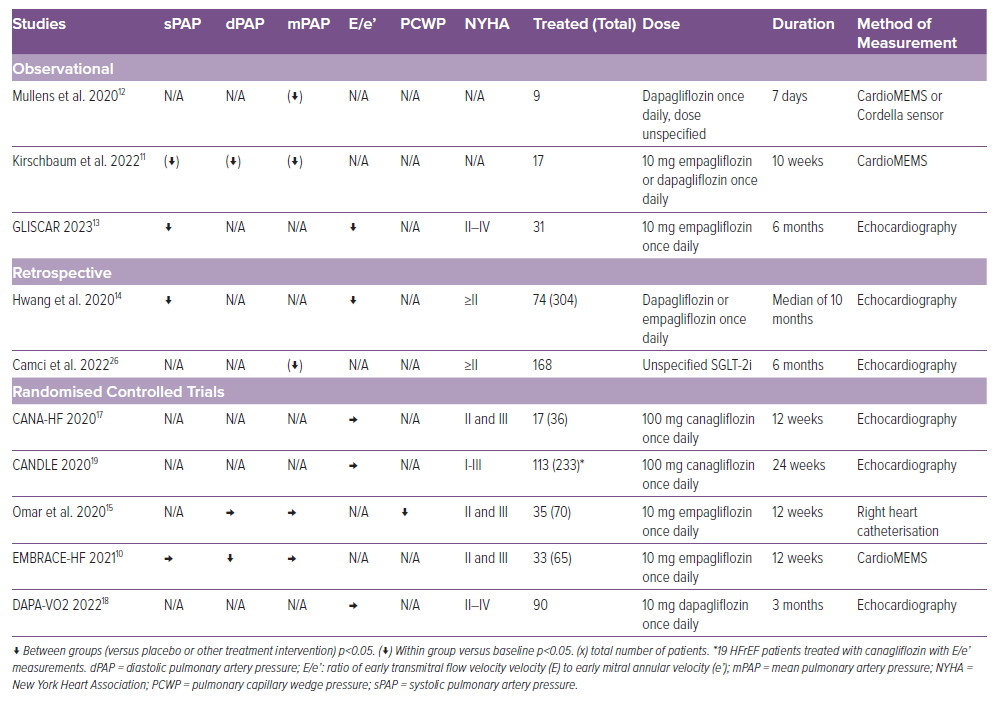
The results across the studies regarding the effect of SGLT-2i on left ventricle filling pressure (LVFP) in HFpEF patients are conflicting (Table 2). In the CAMEO-DAPA trial, dapagliflozin reduced PCWP both at rest and at exercise, measured by right heart catheterisation.16 Sakai et al. found a significant reduction in both E/e’ (lateral, septal and mean) and mitral early E/A ratio.20 Soga et al. did not observe a reduction in E/A. However, both Soga et al. and the EmDia-trial observed a reduction in E/e’.21,22 It should be noted for both studies only around 2/3 of the HF patients were HFpEF. The reduction in E/e’ observed by Hwang et al. was only significant for patients with HFrEF and not HFpEF patients.14 Neither the EXCEED trial, the Muscat-HF trial nor the CANDLE trial observed a decrease in E/e’ or in E/A.19,23,24 The Muscat-HF trial also had a higher than expected proportion of patients with mild HF (97% of patients with HF with a New York Heart Association 2 grading), where less improvement is expected.24 Overall, Oka et al. did not observe a significant decrease in E/e’; however, they did observe a decrease for the subgroup of patients with early diabetes-related cardiomyopathy (DMCMP) defined as extracellular volume fraction ≤30% (a reliable cardiac fibrosis marker).25
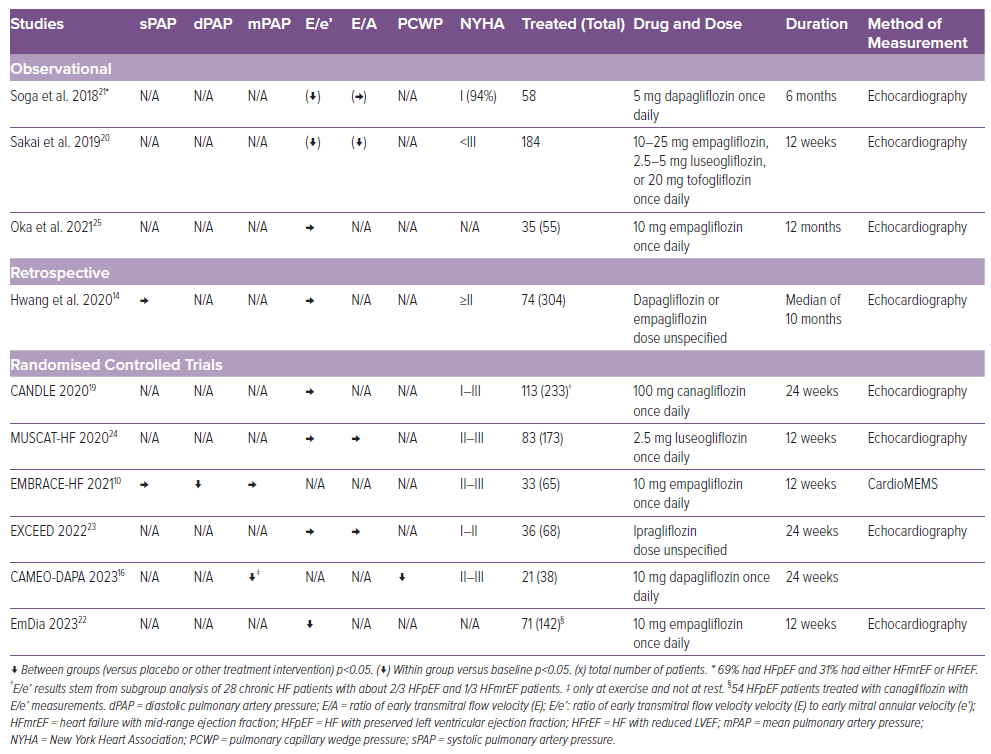
Cardiac Output
Very limited research has been published focusing on changes in cardiac output. Omar et al. measured cardiac index (CI) and PCWP/CI in HFrEF patients and found no effect on neither CI nor PCWP/CI.15 However, Camci et al. did find an increase in CI.26 The CAMEO-DAPA trial investigated cardiac output in patients with HFpEF and observed no changes after treatment with SGLT-2i.16
Systemic Blood Pressure
The effect of SGLT-2i on the systemic blood pressure has been mainly reported as an exploratory variable or as a secondary outcome. The effect in HFrEF patients points towards a small reduction in the systemic blood pressure (Table 3).2,15,27–30
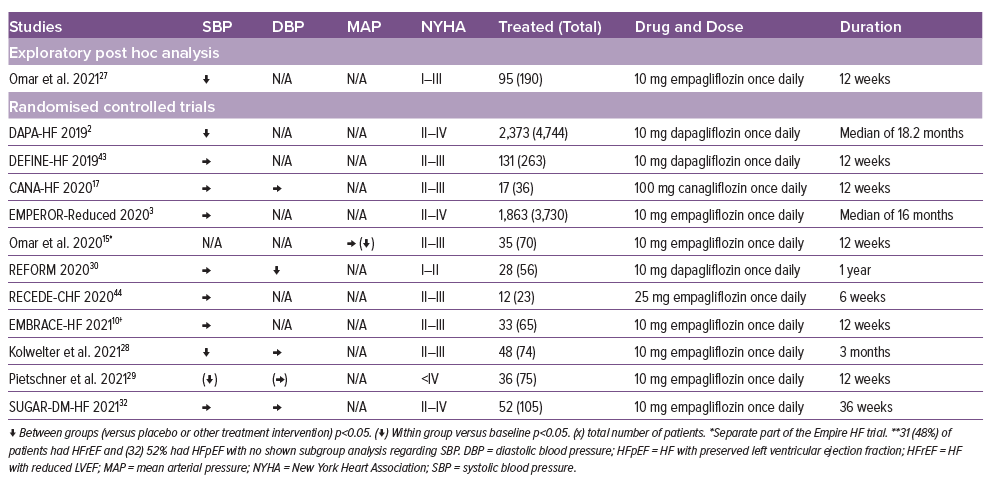
Kolwelter et al. and Pietschner et al. observed a decrease in aortic stiffness. A decrease was observed in central systolic blood pressure (cSBP), central pulse pressure (cPP), forward pulse pressure height (FPH) and reflected pressure pulse height (RPH) after 3 months of treatment.28,29
In HFpEF, the effect of SGLT-2i on SBP has been explored in multiple studies, but only the EMPEROR-Preserved trial, Sakai et al. and the Muscat-HF trial observed a reduction in SBP (Supplementary Table 2).4,20,24 In addition, Sakai et al. found a reduction in diastolic blood pressure. A post hoc analysis of the DELIVER trial observed a reduction in SBP after 1 month, which seemed to diminish over the full-time range of the trial.31
Cardiac Remodelling
The effect of SGLT-2i on LV dimensions and volumes in patients with HFrEF has been investigated in 10 studies (Supplementary Table 3). Seven of the studies demonstrated a decrease in left ventricular end diastolic (LVEDV) and systolic volume (LVESV) and/or left ventricular mass (LVM). The decrease in LV volumes has been observed by multiple studies through the measured reduction in LVESV or LVESV index (LVESVI), LVEDV or LVEDV index (LVEDVI) and left atrial volume (LAV) or LAV index (LAVI).14,27,32,33 Omar et al. used echocardiography to observe a statistically significant reduction compared to placebo in LVESVI, LVEDVI and LAVI after 12 weeks of treatment with SGLT-2i.27 The SUGAR-DM-HF trial, using cardiac magnetic resonance (CMR), also found a decrease in LVESVI and LVEDVI after a treatment period of 36 weeks.32 Likewise, Camci et al. observed reductions in LVESV and LVEDV after 6 months of treatment. These observations are also corroborated by Hwang et al. who observed a reduction in LVEDV after a median treatment period of 10 months and the EMPA-TROPISM trial which used CMR to observe reductions in LVESV, LVEDV and LV sphericity after 6 months of treatment.14,33
The CANA-HF trial investigated the effect of SGLT-2i on cardiorespiratory fitness compared to sitagliptin and in addition had exploratory analyses of cardiac function measured by echocardiography.17 It demonstrated a small increase in LVESVI and no difference regarding LVEDVI. It was terminated early due to guideline changes recommending canagliflozin over sitagliptin and was under powered. No change in LVV or LAVI was observed in neither the GLISCAR study, the REFORM trial nor the DAPA-VO2 trial.13,18,30
The observations regarding a decrease in LVM are ambiguous. Omar et al., Hwang et al., the EMPA-VISIONS trial and the EMPA-TROPISM trial observed reductions in LV hypertrophy, whereas neither the GLISCAR study, the SUGAR-DM-HF trial nor the REFORM trial observed such as decrease.13,14,27,30,32–34 It should be noted that the REFORM trial included patients with fewer symptoms and higher baseline LVEF and did not show any effects on LV dimensions.32
With a decrease in LV volumes it could have been anticipated that LVEF would correspondingly increase.32 This was only found to be the case in the GLISCAR study, the EMPA-TROPISM trial, the CANA-HF trial and in the trials by Hwang et al. and Camci et al.13,14,17,26,33 Interestingly, Omar et al. observed an increase in LVEF in the subgroup naïve to diuretics.27
Nine studies examined cardiac remodelling with SGLT-2i treatment in HFpEF patients (Supplementary Table 4). A potentially deleterious reduction in LVEDV was observed by Hwang et al.14 Oka et al. observed an increase in global longitudinal strain (GLS) for both early and advanced DMCMP but more pronounced for early DMCMP.25 Otherwise, only Soga et al. observed structural changes following treatment with an observed increase in LVEF and in GLS and an observed reduction in LAV and LVMI. However, the EXCEED trial, partly supports the observations as their subgroup analyses revealed a decrease in LVMI in patients aged ≥70 years. As noted earlier, the lack of significant results in the EXCEED trial could be due to low power.23 The Muscat-HF trial observed no effect on remodelling, but this may be due to either low power or the fact that more patients than expected had mild HF.24 Likewise, in the EMPA-VISION trial no effect on remodelling was observed, however, a large percentage of patients were excluded post-randomisation due to COVID-19 restrictions.34
A recent open-label, interventional study of 162 chronic HF patients irrespective of baseline LVEF observed reversal of cardiac remodelling in terms of a decrease in LAVI, LVESV, LVEDV, LVMI and an increase in GLS and LVEF. The study also observed a decrease in SBP, but no effect on E/e’. It is not systematically incorporated in this review due to its clinical characteristics and lack of available subgroup analysis regarding HFrEF and HFpEF.35
Two further studies also examined the effect on the right ventricle (RV). The GLISCAR study and Camci et al. also investigated the effects of SGLT-2i treatment on right ventricular (RV) function and observed improvement in RV systolic longitudinal function evident from a significant increase in tricuspid annular plane systolic excursion (TAPSE).13,26 Camci et al. also found an increase in RV fractional area change (not observed in the GLISCAR study), RV peak systolic S’-velocity (RV S’), and a significant decrease in RV myocardial performance index.26
Discussion
The main findings of this review are trends towards improvements in central filling pressures and systemic blood pressure with only a modest impact on adverse LV remodelling following SGLT-2i treatment. The improvements observed are modest and do not seem to fully explain the magnitude of clinical benefit demonstrated in the large outcome trials. The effect observed appears most evident for HFrEF patients, with most studies demonstrating at least one significantly improved variable in central haemodynamics (PAP and LVFP) and in cardiac remodelling, whereas the results for HFpEF patients were conflicting (Figure 2). Although a reduction in blood pressure has been demonstrated in some clinical outcome trials, the evidence collected in this review does not unequivocally demonstrate this.2,4
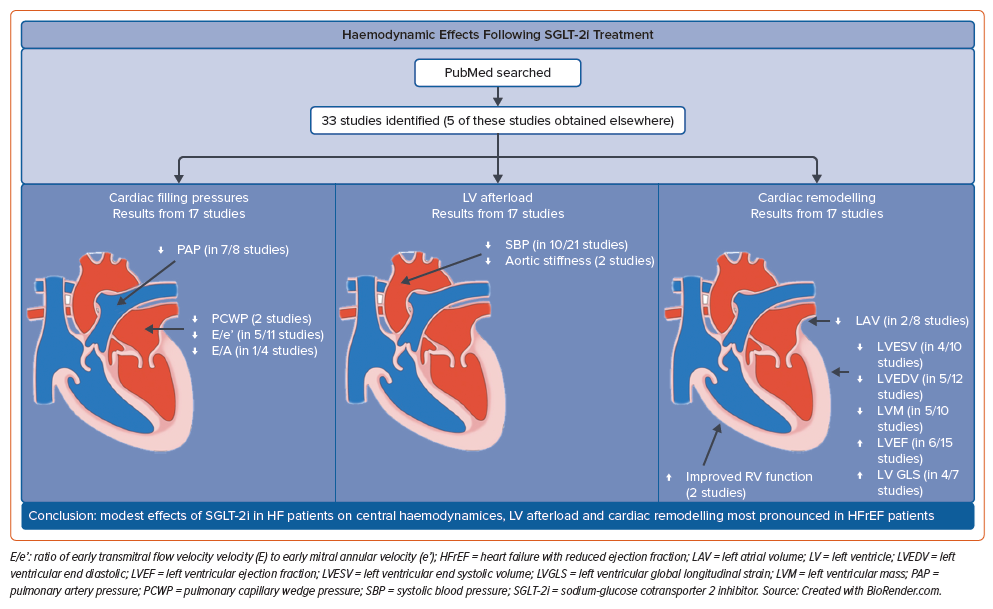
HFrEF and HFpEF differ in aetiology, co-morbidity burden and clinical outcomes, and this may partly explain the different effects of SGLT-2i in the two patient groups. The theorised mode of actions of SGLT-2i are so far still widely debated. Hwang et al. suggest that the more prominent improvements made in HFrEF compared to HFpEF support the notion that the improved cardiac function could in part be due to effective volume reduction and mitigation of the activated neurohormonal axis. This is supported by the notion that SGLT-2i appear to decrease the activity of the renin–angiotensin–aldosterone system (RAAS) as it has been shown to decrease the urinary angiotensinogen-to-creatinine ratio (intrarenal RAAS marker) in type 2 diabetes patients.36
HFrEF patients exhibit higher levels of natriuretic peptides due to the volume overload-induced stretching of the LV and accompanied neurohormonal activation.37,38 Hwang et al. observed a significant decrease in N-terminal pro B-type natriuretic peptide in HFrEF patients, but not in HFpEF patients who also had significantly lower baseline values. Therefore, it could be postulated that the decongestion may drive some of the improvement in cardiac function as it seem to partly explain the more pronounced effects in HFrEF patients. The fast onset of reductions in PAP also suggests a diuretic aetiology behind the decongestion and thus reduction in PA pressures. This reduction seems likely involved in the decrease in mortality and hospitalisation for HF as the risk of adverse outcomes increases significantly even with minor elevations in PA pressures.39 It should be noted that diuretic dosing did not change significantly over the course of the study period in several of the studies reporting diuretic regimens and the reductions in PA pressures observed in the EMBRACE-HF trial continued after 1 week of discontinuation of SGLT-2i treatment, suggestive of a sustained benefit not fully explained by the diuretic effect.2,10–12
The authors of the EMRACE-HF trial proposed that the decrease in PAP could be because of a direct vasodilatory effect on the pulmonary vasculature as SGLT-2i could improve endothelial function or because of a decrease in LVFP.40 A decrease in LVFP has been demonstrated both invasively and non-invasively and seems further supported by RV amelioration, as reported by Camci et al.14–16,20,21,26 A decrease in LV afterload could contribute to a decrease in cardiac filling pressures through improved LV diastolic properties, especially as a decrease in LVM has repeatedly been demonstrated.14,21,27,33 A decrease in afterload could result from both a decrease in aortic stiffness as observed by Kolwelter et al. and a decrease in SBP.28
A reduction in BP without a compensatory increase in heart rate was noted in the EMPA-REG OUTCOME trial as a potential haemodynamic effect of SGLT-2i. In this review, we found differing results in terms of BP changes with SGLT-2i in HFrEF and HFpEF with the DAPA-HF and the EMPEROR-Preserved trials reporting modest lowering of SBP, whereas the EMPEROR-Reduced trial did not report a significant reduction. Sub-studies to both the EMPEROR-Reduced and the DAPA-HF trial explored the interplay between SBP and treatment with SGLT-2i.41 These studies showed that HFrEF patients with a low systemic blood pressure (<110 mmHg) did not have elevated risk of hypotension and in fact tended to have higher absolute risk reduction with SGLT-2i than HF patients with hypertension.41,42 A sub-study to the DELIVER trial investigated the same effect on heart failure with mid-range ejection fraction HFmrEF and HFpEF patients and similarly demonstrated the benefit and safety of SGLT-2i treatment irrespective of baseline SBP.31
The studies included in this review suggest that the impact of SGLT-2i on reversal of cardiac remodelling are modest and most pronounced in patients with HFrEF. Treatment interventions that reduce or reverse cardiac remodelling have been shown to be accompanied by better clinical outcomes in patients with HFrEF. Therefore, it seems likely that the reduced or reversed cardiac remodelling plays a role in the improved HF outcome following SGLT-2i treatment in HFrEF.32 The modest changes in cardiac chamber geometry observed over time with SGLT-2i treatment in both HFrEF and HFpEF further highlights that the improved clinical outcomes with SGLT-2i are not fully explained by changes in LV loading conditions and reverse cardiac remodelling.
Limitations
PubMed was the only searched database and relevant articles might have been missed. Several of the studies included are small and heterogenous regarding control group, blinding, randomisation, treatment duration, dosage, methodology for assessing primary outcome and the number of centres involved. It should be noted that the contradictory findings did not seem to be fully explained by any of these listed differences in design or measurements. Furthermore, this review has not distinguished between whether a variable was a primary, secondary or exploratory endpoint, or which type of SGLT-2i was used in treatment. Finally, this review did not include studies examining the haemodynamic effects of SGLT2 inhibitors in patients treated for acute decompensated HF, such as SOLOIST-WHF and EMPAG-HF.
Conclusion
SGLT-2i treatment in patients with HFrEF and HFpEF results in modest improvements in central haemodynamics, LV afterload and cardiac remodelling. The observed haemodynamic effects are not explained by the diuretic properties of SGLT-2i nor do they appear to fully explain the clinical effect demonstrated in the large outcome trials.