Publications on heart failure (HF) with preserved ejection fraction (HFpEF) date back to 1984–85. Today, approximately 50% of all HF patients demonstrate preserved ejection fraction, and the prevalence of this condition continues to increases annually.1 This rise may be related to the growing incidence of comorbidities, such as hypertension, obesity, type 2 diabetes (T2D), coronary artery disease (CAD), chronic kidney disease (CKD), AF and obstructive sleep apnoea.
It is well known that HF treatment targeting the neurohumoral triad has almost no impact on the prognosis of patients with HFpEF. Over the past few years, there has been an increasing discussion about the potential cardiovascular benefits of sodium-glucose cotransporter 2 inhibitors (SGLT2Is) and glucagon-like peptide-1 receptor agonists (GLP-1RAs).
These medications, which are used primarily for T2D management, have shown beneficial effects on both cardiac and renal diseases. Significant evidence from large, randomised trials has shown that SGLT2Is have a positive impact on heart failure with reduced ejection fraction (HFrEF) as well as on HFpEF.2,3,4,5
This review explores the current data on cardiovascular effects and potential advantages of GLP-1RA therapy in patients with HFpEF.
Mechanism of Action
Glucagon-like peptide-1 (GLP-1) is a member of the incretin family (gastrointestinal hormones released after meals that increase insulin secretion during periods of hyperglycaemia). GLP-1 is a 30-amino-acid polypeptide synthesised by the L cells of the small intestine.6
Glucagon-like peptide-1 receptors (GLP-1Rs) belong to the B family of G protein-coupled receptors. In humans, GLP-1Rs are found in a variety of tissues, including pancreatic islets, the lungs, the kidneys, the gastrointestinal tract, the central nervous system, the vagus nerve, endothelial cells and smooth muscle cells, as well as in atrial and ventricular cardiomyocytes.7
GLP-1RA effects that may be important in patients with HFpEF include glycaemic control, weight loss, reductions in epicardial fat and blood pressure (BP), alleviation of atherogenesis and systemic inflammation and improvement of endothelial function.
The hypoglycaemic effect of GLP-1RAs is achieved by stimulating insulin secretion in pancreatic β cells, suppressing glucagon secretion in pancreatic α cells, and slowing gastric emptying.8
Weight Loss and GLP-1RA Therapy
The use of all types of GLP-1RAs, to a greater or lesser extent, leads to body weight reduction due to decreased appetite and promoting feelings of satiety and stomach fullness, resulting in a lower calorie intake from food.8
The key aspects of weight loss with GLP-1RA therapy are related to GLP-1RA and GLP-1R interaction in the hypothalamus arcuate nucleus and hindbrain; POMC/CART neurons stimulation and NPY/AgRP neurons inhibition; C-Fos neuron stimulation in the rhomboid fossa and the medulla oblongata solitary tract; and secondary stimulation (associated with activation in neighbouring areas, rather than influence on GLP-1Rs) of C-Fos neurons in the thalamic nuclei and CGRP neurons in the parabrachial nuclei.9,10
These actions change eating behaviour: the anticipatory food reward for looking forward to eating certain (delicious) foods decreases, while consummatory food reward (the pleasure offered by eating) increases.8 Thus, it fosters reduction in appetite, food quantity and calorie intake. The energy expenditure lowering in response to the reduction in food intake during GLP-1RA therapy occurs within the first week of therapy.
The Effect of GLP-1RAs on Epicardial Adipose Tissue
GLP-1Rs are expressed in both subcutaneous and visceral adipose tissue; GLP-1Rs appear to be involved in fat cell precursor differentiation.11
A study has shown that GLP-1Rs are increased in the visceral adipose tissue of morbidly obese patients with insulin resistance.12 In high concentrations, GLP-1RAs show lipolytic activity. This was demonstrated in two randomised controlled trials (RCTs) that showed a decrease in epicardial adipose tissue in patients with T2D and obesity on liraglutide and exenatide according to echocardiography and cardiac MRI.13,14
The Effect of GLP-1RAs on Atherogenesis
GLP-1R is expressed in endothelial cells, monocytes, macrophages and smooth muscle cells.
Animal studies have shown that GLP-1RA therapy reduces: reactive oxygen species production; macrophage and monocyte activation by oxidised low-density lipoprotein; activation of adhesion molecules VCAM-1, MCP-1, E-selectin and ICAM-1; and foam cell formation and endothelial cell apoptosis, which reduces the atherosclerotic plaque’s necrotic core.15–17
The GLP-1R stimulation in smooth muscle cells leads to a decrease in both their proliferation and the migration of smooth muscle cells into a plaque.18
GLP-1R activation in endothelial cells results in the expression of more endothelial nitric oxide synthase, providing greater amounts of nitric oxide and consequent vasodilation.19
The Effect of GLP-1RAs on Blood Pressure
A meta-analysis of 16 RCTs showed that use of GLP-1RAs leads to a moderate BP reduction in patients with hypertension.20
Possible mechanisms are: natriuresis, due to increased atrial natriuretic peptide secretion, GLP-1R activation in the proximal nephron tubule and Na+/H+ transporter inhibition (which reduces sodium reabsorption), as well as vasodilation due to nitric oxide-dependent vasodilation (endothelial GLP-1R activation) and direct effects on smooth muscle cells’ GLP-1R.21–23
The Effect of GLP-1RAs on Systemic Inflammation
The ability of GLP-1RAs to reduce systemic inflammation (with decreases in hs-C-reactive protein, TNF-α, interleukin-6 and interleukin IL-1β) has been demonstrated in animal studies, studies on isolated human tissues and RCTs.
These changes result from the direct effect of GLP-1RAs on proinflammatory cytokines production in monocytes/macrophages as it blocks NF-κB, and lowers the formation of pro-inflammatory M1 macrophages.24 As a result, there is a reduction in the atherosclerotic plaque’s macrophage-associated inflammation.
Evidence for GLP-1RAs in HFpEF
Seven RCTs have shown the cardioprotective benefits of GLP-1RAs in patients with T2D, regardless of their cardiovascular risk profiles. According to a meta-analysis by Marsico et al., these studies showed a reduced risk of major adverse cardiovascular events (MACEs; cardiovascular mortality, MI and fatal and non-fatal stroke) (HR 0.88; 95% CI [0.80–0.96]), cardiovascular mortality (HR 0.88; 95% CI [0.79–0.98]), all-cause mortality (HR 0.89; 95% CI [0.81–0.97]), fatal and non-fatal stroke (HR 0.84; 95% CI [0.76–0.94]) and HF hospitalisation (HR 0.92; 95% CI [0.86–0.97]).25 Significantly, only a meta-analysis demonstrated the risk reduction in HF hospitalisation; the data from individual studies did not reveal any statistically significant differences between GLP-1RAs and placebo.
A number of studies dedicated to GLP-1RA efficacy in T2D included patients with chronic HF (CHF) (16.2% in the EXSCEL trial, 23.6% in SUSTAIN-6, 12.2% in PIONEER, 8.6% in REWIND, 20% in Harmony, 22% in ELIXA and 18% in LEADER).26–32 Most studies do not provide left ventricular ejection fraction (LVEF) data, which suggests the presence of both HFrEF and HFpEF. EXSCEL alone reported 22% of patients had reduced LVEF.26
Conflicting results were obtained in subanalyses with regard to reduction of cardiovascular events in patients with CHF. Subanalyses of EXSCEL, SUSTAIN-6 and PIONEER-6 trials suggested that patients with СHF may not necessarily benefit from GLP-1RA therapy. At the same time, in LEADER, the therapy’s effectiveness was not impacted by the presence or absence of CHF.33–35 The effectiveness of therapy in patients with HFpEF alone was not separately assessed in this subanalysis.
Studies of HFrEF patients raised questions about the safety profile of GLP-1RA therapy in this subpopulation. In the LIVE study, which examined the effects of liraglutide in patients with stable HFrEF, no changes in LVEF were observed. However, treatment with liraglutide was associated with a significant increase in serious cardiac side-effects (12 versus three; p<0.05).36 The FIGHT trial, which looked at patients who had recently been hospitalised for acute HF and were treated with liraglutide, found the therapy was associated with higher rates of HF hospitalisations or all-cause deaths than placebo (143 versus 96 events), although the difference did not reach statistical significance.37
The efficacy and safety of GLP-1RAs may vary across the spectrum of HFrEF and HFpEF patients. Recently, the effects of GLP-1RAs in HFpEF patients were investigated in two RCTs. The STEP-HFpEF DM trial enrolled 616 HFpEF patients with obesity (BMI >30 kg/m2) and T2D.38 Patients were randomised to receive subcutaneous semaglutide 2.4 mg weekly or a placebo for 52 weeks. In the semaglutide group, there was an improvement in quality of life according to the Kansas City Cardiomyopathy Questionnaire (KCCQ-CSS), with a mean change in KCCQ-CSS score of 13.7 points with semaglutide and 6.4 points with placebo (p<0.001) and a decrease in body weight (−9.8% versus 3.4%; p<0.001). Patients in the semaglutide group also demonstrated an increase in 6-minute walk test distance (6MWD) (differences between groups: 14.3 m; p=0.008), a decrease in C-reactive protein (CRP; −42.0 versus −12.8%; p<0.001) and N-terminal pro b-type natriuretic peptide (−23.2 versus −4.6; p<0.05). The semaglutide group patients did not have more serious side-effects than those in the placebo group (17.7% versus 28.8%).38
The STEP-HFpEF study included 529 patients with HFpEF and obesity without T2D.39 Patients from the semaglutide group demonstrated a greater 6MWD (21.5 m versus 1.2 m; p<0.001) and improvement in quality of life (KCCQ-CSS), as well as a significant decrease in CRP (−43.5% versus −7.3%; p<0.001), which was accompanied by marked weight loss (−13.3% versus −2.6%; p<0.001). Serious side-effects were reported in 35 (13.3%) patients in the semaglutide group, compared with 71 (26.7%) in the placebo group (p<0.001).39
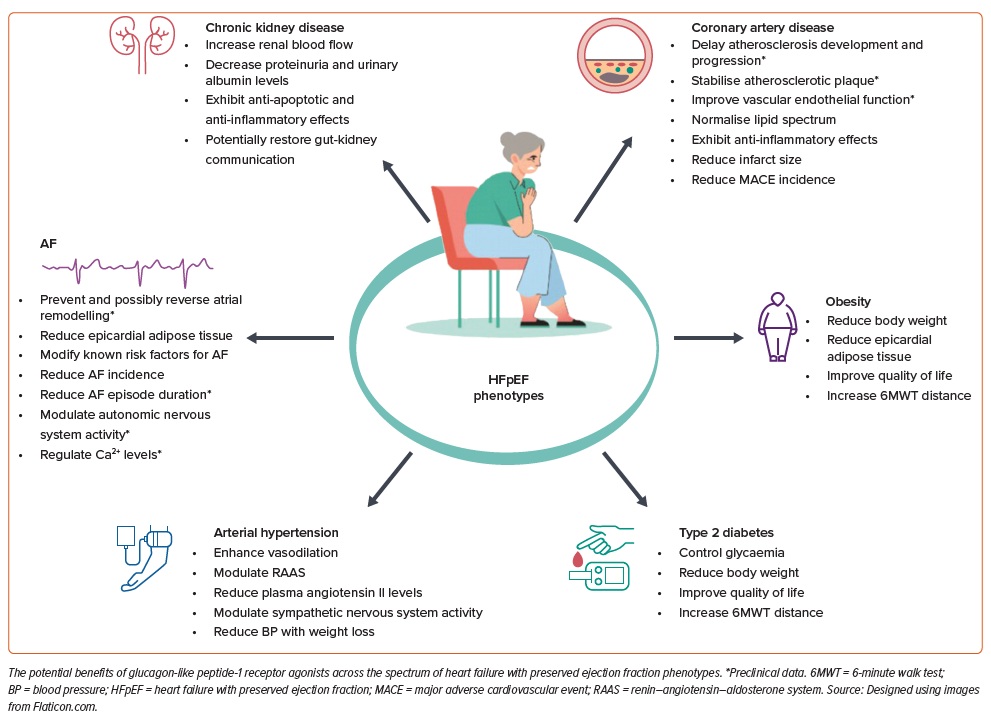
The efficacy of GLP-1RAs may vary between HFpEF phenotypes and also depends on the time of initiation, type of medication and duration of the therapy.
Potential benefits of GLP-1RAs in HFpEF phenotypes are summarised in the Figure 1.
GLP-1RAs and Type 2 Diabetes
T2D is a risk factor for CHF that also increases morbidity and mortality risk in patients with established disease. Trends in increasing T2D and CHF prevalence predict a growing burden of the disease and highlight the need for effective therapeutic strategies.40
A population-based study has traced the relationship between CHF in patients with T2D, irrespective of other comorbidities.41 The risk of developing CHF is 2–5 times higher in patients with T2D than in those without the condition. Younger patients are at higher risk. In the UK Prospective Diabetes Study, patients with T2D aged <65 years had more than a four-fold risk of developing CHF among men and an eight-fold risk among women compared to people without T2D.42
In patients with T2D, hyperglycaemia, insulin resistance and hyperinsulinaemia cause vascular smooth muscle cell proliferation, inflammation, dyslipidaemia and endothelial dysfunction, leading to more rapid CAD onset and progression. CAD is the most common cause of CHF in patients with T2D. T2D patients without CHF symptoms often have subclinical cardiac abnormalities, such as increased left ventricular (LV) myocardial mass, LV hypertrophy, increased left atrium size and impaired LV relaxation.
In patients with T2D, certain issues (inflammation, mitochondrial dysfunction, oxidative stress, changes in calcium homeostasis, increased formation of advanced glycation end products, changes in the myocardial energy substrate as well as autonomic dysfunction), lead to endothelial dysfunction. In case of the coronary microvasculature, it leads to LV diastolic dysfunction.
Advanced glycation end products increase myocardial fibrosis and LV diastolic stiffness and impair cardiac relaxation. Hyperglycaemia activates the renin–angiotensin–aldosterone system (RAAS), leading to angiotensin II and aldosterone overproduction, which causes myocardial hypertrophy and fibrosis. Patients with T2D may develop diabetic cardiomyopathy, which is identified as systolic or diastolic dysfunction in the absence of other causes.40,43
The impact of glycaemic control on clinical outcomes in patients with CHF and T2D was analysed by Lawson et al. Their findings indicated that a reduction in HbA1c levels of >1% significantly decreased hospitalisations (aOR 1.33; 95% CI [1.18–1.49]) and mortality (aOR 1.36; 95% CI [1.24–1.48]).44 Yao’s meta-analysis of 76 RCTs on GLP-1RAs found a consistent HbA1c reduction of 2.10% (95% CI [−2.47, −1.74%).45
All studies that demonstrated a reduction in MACE on GLP-1RA therapy consistently involved patients with T2D. The GLP-1RA cardioprotective impact was accompanied by a significant decrease in HbA1c levels.26,27,29–32
Only one study was dedicated to patients with HFpEF and T2D. The patients in the STEP-HFpEF DM trial saw statistically significant improvements in 6MWT distance and quality of life, as well as reductions in HbA1c level.30
GLP-1RAs and Obesity
According to WHO data, 43% of adults over 18 were overweight and about 16% worldwide were obese in 2022.46 There is a relationship between HFpEF and obesity, with the latter considered not only a significant comorbidity but also a direct contributor to HFpEF. Patients with obesity and HFpEF are classified into a separate phenotype due to a specific clinical presentation and haemodynamics.47
Visceral fat accumulation in patients with obesity causes a number of changes (oxidative stress, activation of the RAAS and gut dysbiosis), resulting in insulin resistance, increased vascular stiffness, T2D, dyslipidaemia, hypertension, coronary atherosclerosis and, ultimately, HFpEF.
Patients with visceral obesity demonstrate increased LV myocardial mass, concentric LV hypertrophy and diastolic dysfunction. Patients with obesity and HFpEF typically have increased pulmonary vascular resistance and mean pulmonary artery pressure, as well as signs of right ventricular dysfunction. Additionally, obese patients have increased circulating plasma volume and lower exercise tolerance with a normal or decreased respiratory reserve.48 Patients with HFpEF and obesity are usually younger than HFpEF patients without obesity. They more often exhibit peripheral oedema, orthopnoea, a higher New York Heart Association (NYHA) functional class for HF and significantly reduced 6MWT distance.47
While lifestyle modifications, regular physical activity and diet are recommended for all HF patients, weight reduction should be a direct therapy goal for patients with HFpEF and obesity. The use of GLP-1RAs in patients with obesity and HFpEF may have the following benefits: weight loss; reduction of epicardial fat; reduction of systemic inflammation; and lower BP.
A meta-analysis from Ansari et al. including 14 RCTs showed that GLP-1RAs significantly reduced weight compared to placebo (average −8.77 kg; 95% CI [−10.98, −6.56]; p<0.01) in obese patients without T2D.49 The most significant effect was observed with tirzepatide and semaglutide at doses of 2.4 mg/week or higher. This study also showed that higher GLP-1RA doses are required for weight loss than for glycaemia normalisation (dulaglutide >1.5 mg/week; semaglutide 2.4 mg/week).
In a study by El Hajj et al., dietary counselling in patients with obesity and HFpEF resulted in weight loss of approximately 7%, an increase in 6MWT distance from 223 m to 267 m (p<0.05), and an improvement in the Minnesota Living With Heart Failure score from 59.9 to 37.06 (p<0.05).50
Some studies have shown the benefit of bariatric surgery in patients with obesity and HFpEF. For example, in a study by Mikhalkova et al. in 12 HFpEF patients who underwent bariatric surgery, an improvement in Minnesota Living With Heart Failure score (p=0.018), a lowering in LV myocardial mass (p=0.001) and a reduction in NYHA functional class of HF were observed at 3 and 6 months after surgery.51
The impact of GLP-1RAs on patients with obesity and HFpEF was recently demonstrated in the STEP HFpEF trial.39 Therapy with semaglutide resulted in significant weight loss, an increase in 6MWT distance, improvement in quality of life (KCCQ-CSS), and a significant reduction in CRP. Similar results were observed in the STEP-HFpEF DM trial.38 The SUMMIT trial (tirzepatide versus placebo in patients with HFpEF and obesity) produced similar results (NCT04847557).
GLP-1RAs and Hypertension
GLP-1RAs are known to have a positive impact on systemic BP levels and, although the precise mechanisms behind their antihypertensive properties are not fully understood, their association with BP modulation has been frequently reported in clinical trials.52
As previously reported, GLP-1RAs promote antihypertensive effects by enhancing vasodilation through nitric oxide production and modulating the RAAS, reducing plasma angiotensin II levels.22,53
In the LEADER trial, which enrolled 9,340 patients with T2D at high cardiovascular risk, liraglutide demonstrated modest reduction in systolic BP (mean difference: −1.2 mm Hg; 95% CI [0.5–1.9]), with a slight increase in diastolic BP (mean difference: +0.6 mmHg; 95% CI [0.2–1.0]).32
Dulaglutide in the REWIND study and exenatide in the DURATION study improved both BP and lipid profiles in patients with T2D.29,54 The STEP-HFpEF trial reported a reduction in systolic BP by an average of 4.9 mmHg in the semaglutide group, compared to a reduction of 2.0 mmHg in the placebo group, in a mean difference of 2.9 mmHg. However, the confidence interval for this difference was −5.8 mmHg to 0.1 mmHg, suggesting that the statistical significance of this effect is borderline.39
In the SCALE trial, which enrolled 3,731 overweight or obese adults, liraglutide 3.0 mg daily was shown to reduce both systolic and diastolic BP, with average delta of −2.8 mmHg (95% CI [−3.56, −2.09) and −0.9 mmHg (95% CI [1.41, −0.37), respectively. The presumed mechanism for this BP reduction is related to liraglutide’s weight loss effects.55
Apart from the weight reduction, GLP-1RAs have been shown to exert antihypertensive effects by promoting diuresis and having additional renal effects. In a composite analysis based on two studies involving 55 healthy volunteers, GLP-1RAs demonstrated modulation of certain autonomic nervous system parameters. The first experiment showed no significant changes in heart rate or BP after GLP-1RA administration, whereas the second experiment revealed a statistically significant increase in muscle sympathetic nerve activity (p≤0.05). These results suggest that GLP-1RAs may modulate sympathetic nervous system activity without markedly affecting cardiovascular parameters in a healthy cohort.56
In a study involving 15 healthy subjects and 16 obese men, GLP-1RA infusion following a 9.9 g salt load significantly increased urinary sodium excretion both in healthy subjects (from 74 ± 8 mmol/180 min to 143 ± 18 mmol/180 min; р=0.0013) and in obese men (from 59 mmol/180 min to 96 mmol/180 min; р=0.015).57
In summary, evidence for the BP benefits of GLP-1RAs mainly comes from experimental studies on patients with T2D and obesity, clarifying potential biological mechanisms for this effect.
GLP-1RAs and AF
AF does not just significantly aggravate the course of CHF but can also directly lead to CHF. In patients with chronic HF and HFpEF in particular, AF prevalence might reach 50%.47 AF results in loss of atrial contribution to LV diastolic filling, RR interval irregularity and atrial myopathy. The most recent studies demonstrate the advantage of sinus rhythm control and maintenance among HFpEF patients.58
GLP-1RAs potentially may reduce the risk of AF onset and progression through direct and indirect effects on atrial remodelling, including a reduction in systemic inflammation, improvement of cardiomyocyte metabolism, autonomic nervous system modulation, regulation of intracellular Ca2+ levels and prevention and possibly reversion of atrial fibrosis.59,60 GLP-1RAs reduce epicardial fat, which is known to play a key role in triggering atrial remodelling in obesity.13,14 AF risk reduction may be related to modification of other traditional risk factors for AF such as obesity, hypertension, atherosclerosis, Т2D and obstructive sleep apnoea.
In the study by Bohne et al., glucagon-like peptide-1 (GLP1) and GLP-1RA therapy in dB/dB mice reduced susceptibility to AF and duration of arrhythmia episodes.60 The study demonstrated GLP1’s ability to prevent atrial electrical and structural remodelling, crucial in new-onset and established AF. GLP1 prevented increases in the atrial effective refractory period, P-wave duration, atrial conduction time, atrial conduction velocities and action potential duration, which were observed in dB/dB mice. In addition, GLP1 demonstrated the potential to prevent and reverse atrial fibrosis. Liraglutide had a similar effect but to a slightly lesser extent, which the authors attribute to differences in doses and treatment approach of the two drugs.60
In a meta-analysis by Shi et al., GLP-1RAs significantly reduced AF events compared to metformin, sulfonylurea, insulin and non-sulfonylurea in patients with T2D and were associated with better outcomes compared to other medications.61 However, only one of the five studies included in the meta-analysis was a randomised controlled study.
A number of studies have demonstrated an increase in heart rate associated with GLP-1RA therapy, likely linked to the effect on the sinus node.36,37 It is debated whether this effect might trigger ventricular and atrial tachyarrhythmias. In the LIVE study, treatment with liraglutide was associated with an increase in heart rate by six BPM, as well as with a significant increase in serious cardiac side-effects (12 versus three; p<0.05), including death from ventricular tachycardia (n=1), ventricular tachycardia (n=3), AF requiring cardioversion (n=4), worsening of CHF (n=1) and acute coronary syndrome (n=3).36 A post-hoc analysis of the FIGHT trial also demonstrated a trend towards an increase in arrhythmic events in patients on liraglutide (21 versus 39; IRR 1.76; 95% CI [0.92–3.37]; p=0.088).37 Both studies were focused on HFrEF patients.
However, two large meta-analyses of RCTs concerning T2D did not show any significant risk for atrial and ventricular arrhythmias or sudden cardiac death associated with GLP-1RAs.62,63 Those meta-analyses did not include the FIGHT and LIVE studies, which included patients both with and without diabetes. In a post-hoc analysis of the REWIND trial, the incidence of de novo atrial arrhythmias in patients with T2D over 5 years was similar in dulaglutide and placebo groups (5.6% and 5.3%, respectively; p=0.59). Patients with atrial arrhythmias had risk factors for occurrence and continuation of arrhythmias: they were significantly older, more often had obesity, a history of AF and LV hypertrophy and were current tobacco users.64 In the STEP-HFpEF study, the incidence of AF and atrial flutter was lower in the semaglutide group than in the placebo group (1.1% versus 3.4% and 0% versus 1.1%, respectively).39
Thus, to date there are insufficient data regarding the effectiveness of GLP-1RAs in reducing the risk of AF onset and progression in HFpEF patients. Data obtained in experimental studies need to be confirmed in larger clinical trials. The STEP-HFpEF results demonstrate a trend towards reduced incidence of AF in the study group.39
GLP-1RAs and Coronary Artery Disease
Epicardial CAD is commonly seen in HFpEF. According to the studies, previous MI is a powerful risk factor for subsequent HFpEF onset. In addition, in recent years, a significant role has been attributed to coronary microvascular dysfunction as one of the pathogenetic factors for HFpEF progression. Data show that up to two-thirds of patients with HFpEF have CAD or coronary microvascular dysfunction including ischaemia, which leads to disruption of LV diastolic and systolic function, development of fibrosis, increased myocardial stiffness and cardiac remodelling.65,66
Preclinical studies have demonstrated that GLP-1RAs might delay atherosclerosis. It has been shown using various atherosclerosis animal models that GLP-1RA therapy is associated with a reduction in atherosclerotic plaques, prevention of aorta intima-media thickening, improvement in vascular endothelial function, reduction of the necrotic zone and an increase of the fibrous component in the plaque and plaque stabilisation.17,67,68
The mechanism behind atherosclerosis prevention is not completely clear. Evidence suggests that this effect is not related solely to weight reduction and better glycaemic control. Possible mechanisms may include anti-inflammatory effects as well as suppression of vascular smooth muscle cell proliferation induced by angiotensin II.17,67
A meta-analysis by Sun et al. addressed the effects of GLP-1RA on the lipid profiles of patients with T2D. GLP-1RA therapy was associated with reductions in total cholesterol, low-density lipoprotein cholesterol and triglyceride levels compared with placebo or other hypoglycaemic agents. The effect varied depending on the dose and duration of action.69 Potential mechanisms include decreased intestinal lipid production, reduced intestinal triglyceride absorption due to lowered secretion of apoB48-containing chylomicrons, smaller postprandial triglyceride secretion and effects on the expression of genes involved in lipid metabolism.70–73
Woo et al. assessed the effectiveness of GLP-1RAs in patients with ST-segment-elevation MI and primary percutaneous coronary intervention.74 Exenatide therapy was associated with lower cardiac enzyme values during the first 72 hours and a reduction in infarct size according to cardiac MRI at 1 month compared with placebo. After 6 months, according to echocardiography, the exenatide group’s E/e’ values were significantly lower than those of the control group (11.2 ± 0.8 versus 13.8 ± 0.6; p<0.05); exenatide use was also associated with better global longitudinal strain (−18.4 ± 0.4% versus −17.1 ± 0.3%; p<0.05) and global circumferential strain (−20.1 ± 0.09% versus −17.1 ± 0.6%; p<0.05). Fewer than one-third of patients had T2D.74
This may partly explain the ability of GLP-1RAs to reduce MACE incidence, which has been demonstrated in research.33–35 However, the mechanisms behind the class effect on atherosclerosis onset and progression require clarification. Normalisation of the lipid spectrum, suppression of the growth of atherosclerotic plaques and their stabilisation, as well as endothelial dysfunction improvement can reduce the risk of both HFpEF onset and progression.
GLP-1RA and Renal Function
With regard to the effects of GLP-1RAs effects on kidney function, CKD is described as one of the HFpEF phenotypes, emphasising significance of understanding the interplay between cardiac and renal function.75 In view of this, GLP-1RAs have been investigated with the expectation of improvements in both cardiac and renal function in patients with CKD and HFpEF.
GLP-1RAs were originally developed for the treatment of T2D. The meta-analysis of the ELIXA, LEADER, SUSTAIN-6, EXSCEL, Harmony, PIONEER 6, REWIND and AMPLITUDE-O trials shows that the extent of HbA1c reduction may serve as a proxy for the renal benefits of GLP-1RA therapy.76 These medications reduce proteinuria and urinary albumin levels, which serve as indicators of kidney damage.77
In a cohort study of 8,922 patients with T2D and a reduced estimated glomerular filtration rate (<30 ml/min per 1.73 m2), 759 were treated with GLP-1RAs and 8,163 with dipeptidyl-peptidase 4 inhibitors (DPP-4is). Over 2.1 years, cardiovascular events were similar between the groups (13% with GLP-1RAs versus 13.8% in DPP-4is; HR 0.88; 95% CI [0.68–1.13]). However, there were fewer kidney events in the GLP-1RA group (38.2 versus 44.2%; subdistribution HR 0.72 95% CI [0.56–0.93]).78
Recent data also show the potential efficacy of GLP-1RAs in renal function in patients without T2D.
GLP-1 enhances renal function by vasodilating the kidney vessels, increasing blood flow and lowering angiotensin II, which are important for controlling vascular tone. It also promotes sodium excretion, especially in people with a high salt intake, which in turn promotes more GLP-1 release. These effects contribute to renal protection and may improve cardiac function in T2D patients with cardiovascular risk. Additionally, there is evidence suggesting that GLP-1 sensitivity could link salt sensitivity to insulin resistance. Mechanistic human studies propose a GLP-1 kidney axis that helps control kidney function and fluid balance.79
In addition to enhancing nitric oxide generation, GLP-1RAs exhibit both anti-apoptotic and anti-inflammatory effects.80
Moschovaki Filippidou et al. studied the renal effects of GLP-1RAs using a mouse model and found that liraglutide improved kidney outcomes by modulating T-cell activity.81 Similarly, Chien et al. discovered that exendin-4 enhances arteriovenous fistula function in rats with CKD by upregulating eNOS/HO-1.82
The direct impact of GLP-1RAs on significant renal outcomes remains uncertain. However, ongoing research, such as the FLOW trial, which had been comparing GLP-1RAs to a placebo in patients with T2D and CKD, was recently halted prematurely due to their effectiveness. Therefore, the positive cardiovascular effects of GLP-1RAs might be partially attributed to their ability to protect the kidneys, potentially restoring communication between the gut and kidneys.79
While the direct renoprotective mechanisms of GLP-1RAs are still under investigation, their ability to enhance natriuresis and suppress pathogenic angiotensin II production presents a valuable therapeutic strategy in HFpEF complicated by CKD.
Conclusion
Emerging evidence underscores the cardioprotective effects of GLP-1RAs, which make them potentially useful for different subpopulations of patients with cardiovascular diseases, including those with HFpEF.
Early results from preclinical and clinical studies have shown the potential benefits of GLP-1RAs in improving glycaemic control, weight management, endothelial function, atherosclerotic plaque stability, systemic inflammation, renal function, BP levels and AF risk.
A question remains as to whether this therapy is effective in patients with existing HFpEF or if its primary benefit lies in impacting risk factors and associated cardiovascular diseases that contribute to the development of HFpEF. Therefore, the efficacy and specific benefits of GLP-1RAs in HFpEF require further investigation.
Future research should aim to clarify the GLP-1RA-specific benefits and mechanisms in HFpEF, which would help to integrate them more effectively into therapeutic regimens.