Heart failure (HF) is a clinical syndrome with different underlying aetiologies rather than being a specific disease. Traditionally, HF has been defined as a condition where there is a reduced ability of the heart to pump and/or fill with blood, or alternatively an inadequate cardiac output caused by a structural or functional abnormality, or adequate cardiac output secondary to compensatory neurohormonal activation and increased left ventricular filling pressure. Despite different definitions of HF, left ventricular ejection fraction (LVEF) has generally been viewed as the cornerstone of HF diagnosis, characterisation, prognosis and treatment selection.1–3 Natriuretic peptides, which are produced by the heart in response to increased wall inflammation and stress, also provide diagnostic and prognostic information for patients with HF.4 The risk of adverse outcomes is also predicted by elevated natriuretic peptides in patients without HF.5
A universal definition and classification of HF was proposed in 2021. HF was defined as a clinical syndrome with symptoms and/or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.4 The stages of HF were also revised as at risk for HF (stage A), pre-HF (stage B), symptomatic HF (stage C) and advanced HF (stage D). Finally, HF classification based on ejection fraction (EF) ranges was revised, including HF with reduced ejection fraction (HFrEF; EF ≤40%), mildly reduced ejection fraction (HFmrEF; EF 41–49%), and HF with preserved ejection fraction (HFpEF; EF ≥50%). Additionally, a new entity, HF with improved EF (HFimpEF; baseline LVEF ≤40%, a ≥10 point increase from baseline LVEF, and a second measurement of LVEF >40%), was introduced to account for the dynamic and changing clinical trajectories of HF syndrome and the increasingly common scenario where EF improves substantially with treatment.6
HF is considered a pandemic affecting an estimated 64 million people worldwide.7 It is projected that the prevalence of HF will increase due to the ageing of the population. Most recent projections for the US suggest an increase in the prevalence of HF by about 46% from 2012 to 2030, with a corresponding increase in healthcare costs of about 127%.8
Epidemiology studies on HF have many limitations related to differences in definitions, population selection and lack of data from some geographical areas. In addition, most studies rely on administrative data, including International Classification of Disease (ICD) codes, which may be lacking for a significant proportion of HF patients and does not include EF data, self-reported data, which requires that the patients know their diagnosis, or hospital records, which cannot capture patients receiving care in outpatient settings.9 However, despite these limitations, we aim to provide a contemporary assessment of the burden of HF, providing data about its prevalence, incidence and outcomes.
Prevalence
Europe and Northern America
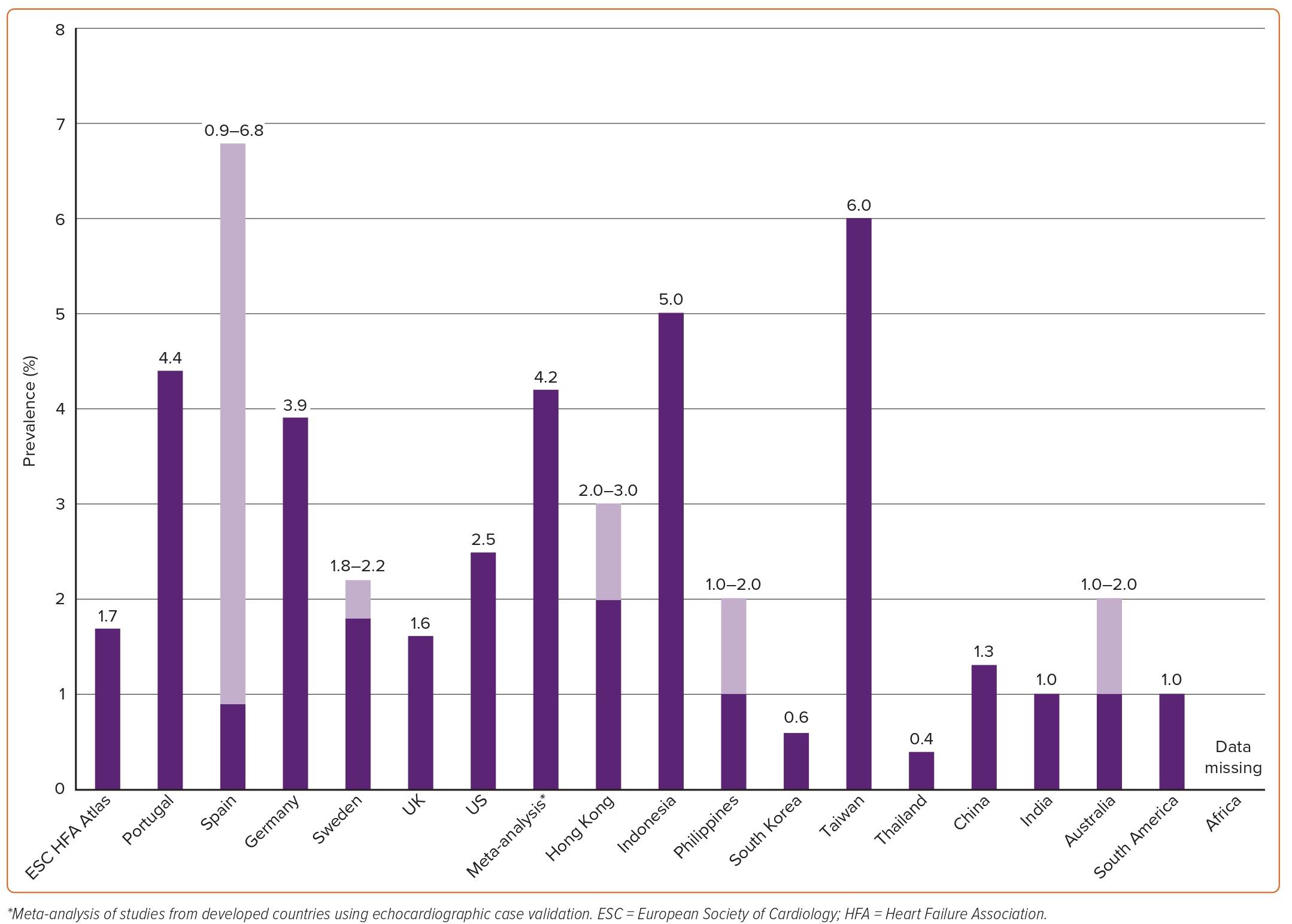
A summary of estimated prevalence of HF across different countries is shown in Figure 1. In the 2019 European Society of Cardiology (ESC) Heart Failure Association (HFA) atlas, data on prevalence were available for 13 (31%) of the participating countries. The median crude prevalence of HF per 1,000 people was estimated at 17, ranging from ≤12 in Greece and Spain to >30 in Lithuania and Germany.10 The estimated prevalence of HF in Germany based on healthcare claims data of over 3 million inhabitants from 2009 to 2014 was 4%.11 In a population-based study of 4 million individuals from the UK, the age- and sex-adjusted prevalence of HF was 1.6% from 2002 to 2014.12 Although this prevalence was stable between 2002 and 2014, the absolute number of patients with HF increased by 23%. The 2021 American Heart Association Heart Disease and Stroke Statistics (NHANES) reported a prevalence of HF of about 2.5% based on self-reported data.8
A meta-analysis based on echocardiographic screening studies from 1997 to 2014 in the general population from developed countries reported a prevalence of any HF of 11.8% in people ≥60 years and about 1% among those <60 years.13 Previously unrecognised cases were also included. Considering that in developed countries about 30% of the adult population is estimated to be 60 years or older, extrapolation of these findings would result in an estimated prevalence of HF of 4.2% (11.8 × 0.30 + 1.0 × 0.70 = 4.2) in the adult population.14 This prevalence is about twice as high as the often-reported estimate of 2% for HF in the adult population at large. Furthermore, the difference between 4% and 2% illustrates that HF may remain undetected in over half of all cases.
The prevalence of HF also varies depending on LVEF-based phenotypes. In the ESC Long-Term Registry (ESC-HF-LT), 60% of patients were classified as HFrEF (EF <40%), 24% as HFmrEF (EF 40–49%) and 16% as HFpEF (HF ≥50%).15 Among patients enrolled in the nationwide Swedish HF registry in 2005–18, 53% had reduced EF, 23% mildly reduced EF, and 24% preserved EF.16 In the OPTIMIZE-HF registry from the US, 49% had HFrEF (EF <40%), 17% had HFmrEF (EF 40–50%), and 24% HFpEF (EF >50%).17 In the GWTG-HF study, including the Medicare population, 39% had HFrEF (EF <40%), 14% HFmrEF (EF 40–50%), and 47% HFpEF (EF ≥50%).18
Interestingly, studies indicate an increase in the prevalence of HFpEF while the prevalence of HFrEF seems to be stable or even declining. In a study of consecutive patients hospitalised with HF at Mayo Clinic Hospitals in Olmsted County, Minnesota, the proportion of the 6,076 patients represented by HFpEF increased from 38% to 54% between 1987 and 2001.19 In the Swedish HF Registry, the overall proportion of HFrEF in 2000–2004 versus 2013–2016 decreased (60% versus 49%) whereas the overall proportion of HFpEF increased (20% versus 30%).20
Asia, Australia, South America and Africa
A summary of the estimated prevalence of HF across different countries is shown in Figure 1. There is a concerning lack of epidemiological data from countries outside Europe and North America, especially from lower and middle-income countries, even though these are estimated to carry 80% of the cardiovascular disease burden.21 HF prevalence has been estimated to be 2–3% in Hong Kong, 5% in Indonesia, 1–2% in the Philippines, 0.6% in South Korea, 6% in Taiwan and 0.4% in Thailand.22 In the China Hypertension Survey, prevalence of HF was 1.3%, of whom 40% had HFrEF (EF <40%), 23% had HFmrEF (EF 40–49%) and 36% had HFpEF (EF ≥50%).23 There are no population-based studies in Japan on the epidemiology of HF but one report estimated a prevalence of left ventricular dysfunction of 0.8% among outpatients.24 In a multicentre cohort study from Japan including patients hospitalised for HF between 2013 and 2014, 36% had HFrEF (EF <40%), 21% had HFmrEF (EF 40–49%), and 43% had HFpEF (EF ≥50%).18 The prevalence of HF in India is estimated to be about 1%.25 Prevalence estimates in Australia range between 1% and 2%.26
In a systematic review, including studies between 1994–2014 from Latin American and Caribbean populations (most studies had been conducted in South America [92%] and mainly in Brazil [64%]), the prevalence of HF was estimated at 1%.27 To date, there are no population-based studies estimating prevalence and incidence in northern and sub-Saharan Africa. In a study from a hospital in Soweto, South Africa, serving 1.1 million people, 1,960 patients presented with HF in 2006 (163 per month), of whom 43% had newly diagnosed HF and 48% of these had HFpEF defined as a LVEF ≥45%.28
Incidence
Europe and Northern America
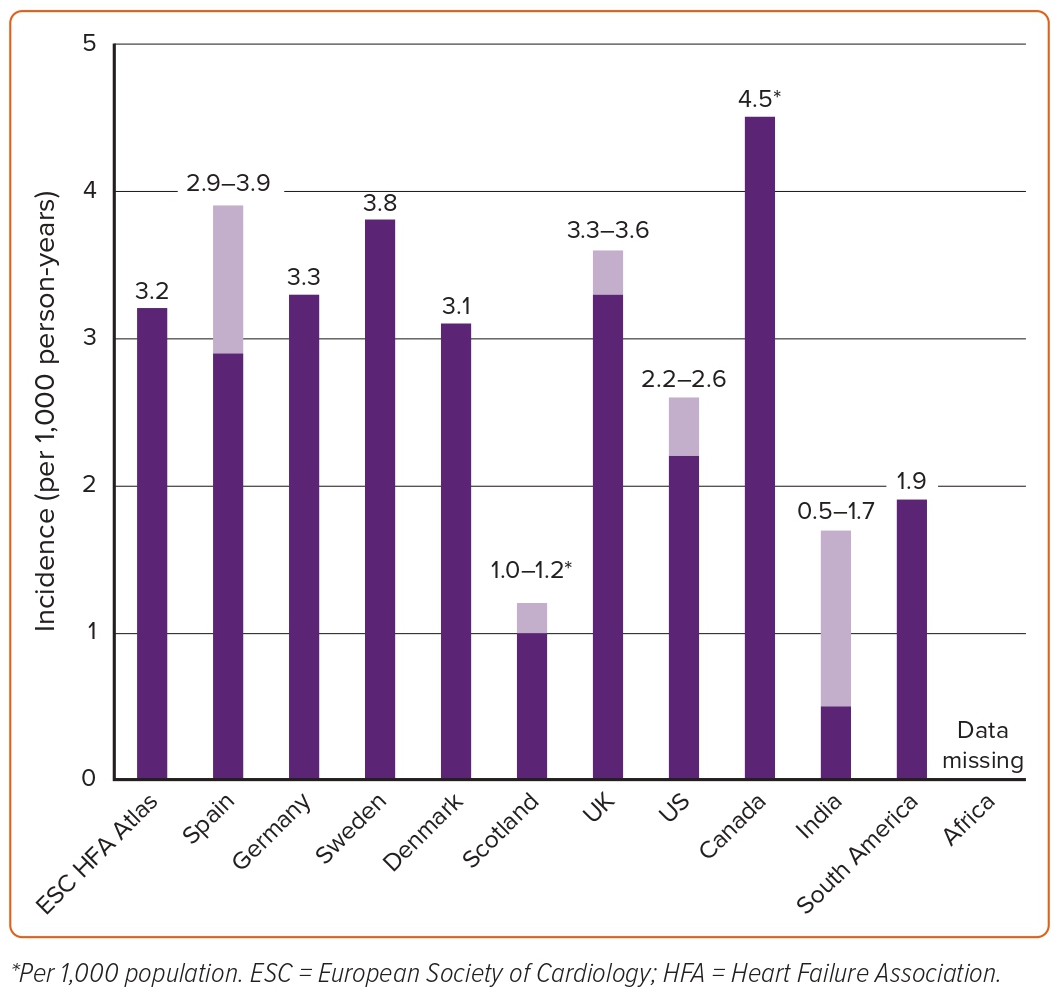
A summary of the estimated incidence of HF across different countries is shown in Figure 2. The reported incidence of HF in European countries and the US ranges widely from one to nine cases per 1,000 person-years depending on the population studied and the diagnostic criteria used. In developed countries, incidence rates have stabilised between 1970 and 1990 and are now thought to be decreasing. Crude incidence statistics were available for 12 (29%) of the participating countries in the ESC HF atlas.10 The median annual incidence of HF per 1,000 person-years was 3.20 cases (ranging from <2 in Italy to ≥6 in Estonia and Germany). In the PREVEND study of 8,592 people in a Dutch community, the incidence rate of HF between 1998–2010 was 3.7 per 1,000 person-years in men and 2.4 per 1,000 person-years in women.29 Among these, 34% were classified as HFpEF (LVEF ≥50%) and 66% as HFrEF (LVEF <40%). Of note, only eight patients had LVEF 41–49%; hence, this category was excluded from the analysis. In a population-based study from the UK including more than 4 million people, a decline of 7% in the incidence of HF was observed between 2002 and 2014 from 3.6 to 3.3 per 1,000 person-years.12 These findings seemed to be largely driven by a decline in the incidence of HF in people aged 60–84 years. However, the incidence remained stable or increased in younger people (<55 years) and the very old (>85 years). In a national sample of hospitalised patients in Denmark, similar trends were observed in the incidence of HF between 1995 and 2012.30
In an analysis from the Cardiovascular Lifetime Risk Pooling Project, the incidence of HF was 7.9 per 1,000 person-years in the Chicago Heart Association detection project in industry and 6.0 per 1,000 person-years in the ARIC study after the index age of 45 years.31–34 However, the incidence of HF was much higher – 21.1 per 1,000 person-years – after the index age of 65 years as observed in the Cardiovascular Health Study.35 In another pooled analysis of the Cardiovascular Health Study and the Framingham Study for participants who were ≥60 years of age and free of HF, the age- and sex-standardised HF incidence rates for 1990–99 and 2000–09 were overall similar at 19.7 and 18.9 per 1,000 person-years, respectively.36 However, divergent trends of decreasing HFrEF and increasing HFpEF incidence were observed. Although HFrEF incidence declined more in men than in women, men had a higher incidence of HFrEF than women in each decade, whereas incident HFpEF increased in both men and women. In contrast, a decline in the incidence of HF was observed in the Olmsted County cohort where the age- and sex-adjusted incidence of HF declined from 3.2 to 2.2 cases per 1,000 person-years between 2000 and 2010.37 The decline was greater in women (43%) than in men (29%) and greater in HFrEF (45%) than in HFpEF (28%).
Asia, Australia, South America and Africa
A summary of estimated incidence of HF across different countries is shown in Figure 2. The incidence of HF in India is estimated to be at least between 0.5 and 1.7 cases per 1,000 person-years, for a total of 492,000 to 1.8 million new cases per year.38 However, the age-specific incidence for India is unknown. In a study including 43 Australian general practices between 2013 and 2018, the age-standardised annual incidence of HF was 3.5 cases per 1,000 person-years.39 The estimated incidence rate of HF was 1.9 per 1,000 person-years in South America.27 Studies on the incidence of HF in Africa are lacking. A summary of estimated incidence of HF across different countries is shown in Figure 1.
Demographic and Clinical Characteristics
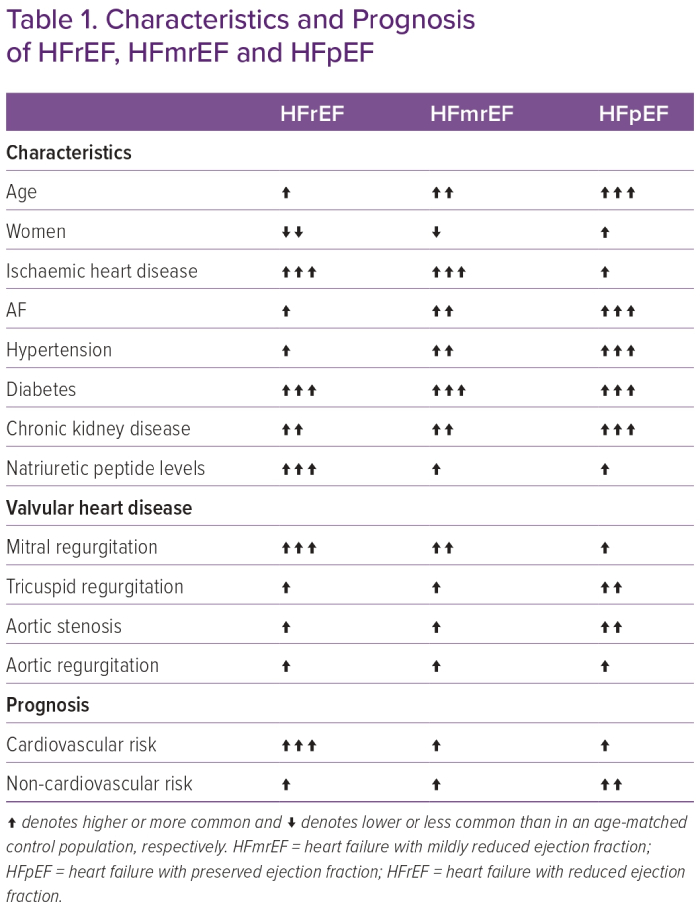
Demographics and clinical characteristics in HF have been shown to differ considerably between LVEF-based HF categories (Table 1). Patients with HFpEF compared with HFrEF are more likely older, women, have a higher prevalence of hypertension, higher mean pulse pressure, obesity, AF and anaemia and suffer more often from comorbidities, such as chronic kidney disease, chronic pulmonary disease, valvular heart disease and cancer, whereas coronary artery disease is the main determinant of HFrEF.15,40–49
In recent years, HFmrEF has been recognised as a potentially distinct entity sharing features with both HFpEF, such as hypertension, milder HF symptoms, lower levels of natriuretic peptides and accordingly lower CV event rates, including HF, and worsening of function and HFrEF, such as a higher prevalence of coronary artery disease and less frequent chronic kidney disease.42,46,48,50–52 The totality of the evidence suggests that in terms of pathophysiology, clinical characteristics, and response to therapy, HFmrEF resembles, on average, more HFrEF than HFpEF.3,4,53
Although valvular heart disease is shown to be common in HFpEF, most available data are from single-centre studies, clinical trials with narrowly selected populations or population-based studies in which patients were not yet diagnosed with HF.54–62 In addition, they lack within-cohort comparisons of the characteristics and consequences of all valvular heart diseases with regards to LVEF-based categories. However, in two studies from the ESC-HF-LT registry with echocardiographic data on LVEF and moderate-to-severe valvular heart diseases, HFpEF seemed to be distinct from HFmrEF and HFrEF with isolated tricuspid regurgitation and aortic stenosis being more prevalent in HFpEF, mitral regurgitation more prevalent with HFrEF and aortic regurgitation having a similar distribution across all HF categories.63,64
Most studies describing HF characteristics have been performed in North America and Europe, although regional differences in phenotypes of HF patients are likely to exist due to different aetiologies, comorbidities, economic and healthcare systems. In the INTER-CHF study, patients with HF in Africa and Asia were younger than in other regions and more likely to be men.65 Ischaemic heart disease was the most common HF aetiology in all regions except Africa where hypertensive heart disease was most common. In the prospective ASIAN HF registry recruiting patients from south Asia (India), south-east Asia (Thailand, Malaysia, the Philippines, Indonesia, Singapore) and north-east Asia (South Korea, Japan, Taiwan, Hong Kong, China), striking regional differences were observed in patient characteristics.66 South-east Asians had the highest burden of comorbidities, particularly diabetes and chronic kidney disease, despite being younger than north-east Asian participants. However, the 23,000-patient global CHF registry (G-CHF), suggests that quality of life in HF is universally poor across the globe and that poor quality of life is a predictor of increased risk of HF hospitalisation and all-cause mortality.67
Outcomes
Europe and North America
Although outcomes in HF in Europe and North America have been extensively studied, estimates of the mortality of HF vary considerably depending on the age and comorbidity profile of the population studied, definitions of HF and among inpatients and outpatients. For instance, mortality rates are higher in observational studies in contrast to clinical trials, which have included outpatients who are younger with a lower comorbidity burden. Outcomes also differ across LVEF-based HF phenotypes.
In the ESC-HF-LT registry enrolling 12,440 patients from 21 European and/or Mediterranean countries between 2011 and 2013, 1-year mortality rates differed between acute and chronic HF (23.6% versus 6.4%) and across countries (21–36.5% for acute HF and 6.9–15.6% for chronic HF).68 In addition, 1-year mortality rates in outpatients differed between HFrEF and HFpEF (8.8% versus 6.3%), while patients with HFmREF experienced intermediate rates (7.6%).15 In acute HF, HFrEF is more severe and has greater in-hospital mortality. Post-discharge, HFrEF has greater CV risk, HFpEF greater non-CV risk, and HFmrEF lower overall risk.41 In the nationwide Swedish HF Registry, crude 1-year mortality rates among 42,061 outpatients were 15.4% in HFrEF, 17.4% in HFpEF and 14.2% in HFmrEF.69 However, the covariate-adjusted risk of 1-year mortality was higher in HFrEF compared with HFpEF (HR 1.26, 95% CI [1.17–1.35]). Similarly, the large MAGGIC study, pooling data from 30 observational studies and clinical trials, reported that patients with HFpEF had a 32% lower adjusted risk of 3-year mortality compared to their HFrEF counterparts. In the ECHOES study including 6,162 patients at a mean age of 64, 10-year survival was 27% for those with definitive HF and 75.4% for those without HF.70 Stratifying by LVEF category, 10-year survival was 76% for patients with LVEF >50%, 48% for LVEF 40–50% and only 31% for those with LVEF <40%. In the GWTG-HF registry of 39,982 patients hospitalised for HF between 2005 and 2009 in the US, a 5-year mortality rate of 75% was reported and mortality rates were similar in patients with HFrEF (75.3%) and HFpEF (75.7%).71 The OPTIMIZE-HF study enrolling 20,118 patients with HFrEF and 21,149 with HFpEF (EF ≥40%) reported a higher in-hospital mortality in HFrEF (3.9%) than in HFpEF patients (2.9%). However, the 30–60-day mortality rates were similar (9.8% versus 9.5%) in HFrEF compared to HFpEF. No differences in outcomes were observed when analysis was stratified according to HFpEF (EF >50%) and HFmrEF (EF 40–50%).17
In a recent systematic review of 60 studies across high-income countries including 1.5 million patients, pooled survival rates at 1 month, 1, 2, 5 and 10 years were 95.7%, 86.5%, 72.6%, 56.7% and 34.9%, respectively.72 The 5-year survival rates improved between 1970–79 and 2000–09 from 29.1% to 59.7%, likely reflecting improved treatment of acute MI and evidence-based and effective treatment options for HF. In the Olmsted County study, survival rates after HF diagnosis improved during the early 1990s and early- to mid-2000s but seemingly levelled off thereafter, possibly reflecting the transition from HFrEF to HFpEF for which effective evidence-based strategies are still largely lacking, and the increasing comorbidity burden in HF.37
Outcomes in HF may fluctuate and change over time. It has been reported that after the initial months following diagnosis of HF, outcomes might improve due to implementation of guideline-directed medical therapy.73 Therefore, repeat assessment of the risk of death from HF should be considered for optimal patient care.
Asia, Australia, South America, and Africa
In the prospective ASIAN HF registry, all-cause mortality for the whole population was 9.6% at 1 year and higher in patients with HFrEF (10.6%) than in those with HFpEF (5.4%).66 One-year, all-cause mortality was significantly higher in south-east Asian patients (13.0%), compared with south Asian (7.5%) and north-east Asian patients (7.4%). In the prospective China-HF registry, in-hospital mortality was 4.1% and significantly higher in patients with HFrEF (4.0%) versus HFpEF (2.4%).74 In the Japanese Cardiac Registry of Heart Failure in Cardiology, in-hospital mortality was higher in HFpEF patients (6.5%) compared with HFrEF patients (3.9%).75 Similarly, 1-year mortality rates were also higher in HFpEF (11.6%) than in HFrEF (8.9%). In a meta-analysis of 12 studies enrolling 67,255 patients hospitalised for HF between 1990 and 2016 in Australia, the pooled estimated 30-day and 1-year mortality rates were 8% and 25%, respectively.76
In the INTER-CHF prospective cohort study of 5,823 patients, overall mortality was 16.5%: highest in Africa (34%) and India (23%), intermediate in south-east Asia (15%), and lowest in China (7%), South America (9%) and the Middle East (9%).65 Notably, these regional differences remained even after multivariable adjustment. Patients in Africa, India and south-east Asia were on average 10 years younger than those in South America and China but had higher mortality rates. In the REPORT-HF, a global registry enrolling patients during hospitalisation for acute HF from 44 countries on six continents, patients from eastern Europe had the lowest 1-year mortality (16%) and those from eastern Mediterranean and Africa (22%) and Latin America (22%) had the highest.77 A large inter-country variation was observed, ranging from 10% in Bulgaria to 32% in Indonesia. Age-adjusted and HF diagnosis-adjusted mortality (new onset versus chronic HF) were higher in patients from lower-income countries (26%) compared with middle-income (20%) and higher-income (17%) countries. Patients from regions with greater income inequality had worse mortality.
Causes of Death
As HF is a syndrome of many underlying causes or conditions leading to cardiac impairment, estimating the number of deaths attributable to HF as the actual cause of death is difficult. Also, cause-specific death and readmission in most registries are obtained from International Classification of Diseases codes or death certificates, which are inherently subject to misclassification.
In the ESC HF-LT registry, mortality at 1 year was mainly due to CV death which was more frequent in HFrEF (53.5%) versus HFmrEF (50.6%) versus HFpEF (47.2%). Conversely, non-CV mortality at 1 year was lower in HFrEF (20.1%) versus HFmrEF (27.8%) versus HFpEF (30.7%).78 In the ECHOES study of causes of death at 10 years follow-up, 44% of deaths were due to CV or cerebrovascular disease, 21% were due to respiratory disease, 21%, due to cancer and 14% due to other causes.70 Definitive HF was the cause of death in 32% of those who had HF with LVEF <40%, 19% of those who had HF with LVEF >40% and 10% of those who had neither HF nor left ventricular systolic dysfunction. In the GWTG-HF registry, between 2005 and 2008, patients with HFrEF had the greatest percentage of deaths caused by CVD (66%) as compared with HFpEF (53%).71 In competing risk analysis, patients with HFrEF had a 26% increased risk of CV death at 1-year follow-up compared with patients with HFpEF. However, the percentage of death attributed to HF was similar in HFrEF and HFpEF (11% versus 10%).
In a subset of patients from the Framingham Heart Study, causes of death were adjudicated by an expert panel for 463 participants with HF who died between 1974 and 2004 and for whom LVEF and detailed death reports were available.79 Overall, 62% of underlying causes of death were CV, with a large proportion of underlying causes attributable to CHD (25%). Progressive pump failure was the major non-CHD cause of cardiovascular death (16% of all underlying causes). Respiratory disease (infectious and non-infectious) was the leading underlying cause of non-CV death (10%), followed by cancer (9%). The underlying causes of death were, to a greater extent, cardiovascular in subjects with HFrEF (70%) than HFpEF (45%). HFrEF increased the odds of CV death more than threefold in men and twofold in women. In the INTER-CHF study, cardiac deaths (46%) were more common than non-cardiac deaths (16%) and deaths from an unknown cause (38%).80
Some studies have reported a shift in the distribution of causes of death over time. In Olmsted County, the proportion of deaths occurring within 5 years of incident HF that were categorised as CV decreased from 74% in 1979–84 to 51% in 1997–2002.81 When stratifying by preserved or reduced LVEF, the proportion of CV deaths decreased mainly in patients with HFpEF.82 In contrast, in a Spanish cohort of 1,876 patients with LVEF <50%, CV deaths decreased significantly over time in patients from 83% in 2002 to 34% in 2018. The decrease in CV death was mainly explained by the decrease in risk of sudden cardiac death, without any change in deaths from MI or stroke and an increase in cancer as a mode of death in HF. In a recent analysis of patients with HFrEF (LVEF ≤40%) from 12 clinical trials spanning the period from 1995 to 2014, a 44% decline in the rate of sudden cardiac death was observed, which paralleled the increasing use of evidence-based medical therapy known to reduce the incidence of sudden death. In a population-based retrospective study from the UK of patients with a first diagnosis of HF between 2002 and 2013, the risk of CV death declined by 27% over time, which was offset by an increase in risk of non-CV death by 22%, with cancer, respiratory conditions and infections being the major non-CV causes of death.83
Hospitalisations
In an analysis from the US National Inpatient Sample Database, HF was consistently among the three most common causes of hospitalisation between 2005 and 2018.84 Also, there was a trend towards increase in hospitalisations for HF during this time, with HF becoming the second most common cause of hospitalisations in 2018. In patients aged >65 years, HF is the most common cause of hospitalisation.85 Patients with HF have the highest 30-day readmission rates (20–25%) compared to patients with other diagnoses. Within 5 years from the initial HF diagnosis, 83.1% of the subjects in the Olmsted County cohort were hospitalised at least once and 66.9% were hospitalised more than twice.86 HF and other CV causes contributed to 16.5% and 21.6% of the hospitalisations, while non-CV causes contributed to most of the hospitalisations (61.9%). Total hospitalisation rates were similar regardless of LVEF, with some evidence of a higher rate of CV hospitalisations among HFrEF offset by a higher rate of non-CV hospitalisations among HFpEF patients.37 In contrast, the rates of total hospitalisation rates at 1 year in the ESC-HF-LT registry were significantly higher in HFrEF patients (31.9%) compared with HFpEF (23.5%) and HFmrEF patients (22%). A similar pattern was observed for 1-year HF-hospitalisation rates, which were higher in HFrEF than HFpEF and HFmrEF (14.6%, 9.7% and 8.7%, respectively).15 Similarly, in the CHARM programme, after adjustment for baseline differences, HFrEF was associated with a 42% increased risk of HF hospitalisations when compared with HFpEF.34 These differentiations may possibly reflect the highly selected populations followed in specialised HF centres and clinical trials, respectively. However, since the case mix of HF is changing, with a larger proportion of patients with HFpEF compared to HFrEF, the proportion of HF hospitalisations for HFpEF seems to be increasing as well. In the GWTG-HF cohort, the proportion of patients admitted for HF who had HFpEF, increased from 33% in 2005 to 39% in 2010.71
During the 1990s, a peak in the number of HF hospitalisations was observed in several developed countries followed by a decline in recent decades. In Denmark, age-adjusted hospitalisation rates decreased between 1983 and 2012 by 25% for women and by 14% for men.87 The decrease reflected an average annual 1% increase between 1983 and 2000 and a 3.5% decline thereafter. In an analysis of the National Inpatient Sample from the US, age-adjusted HF hospitalisation rate decreased by 30.8% from 2002 to 2013 but substantial variations were observed across different races and ethnicities, such as black patients having a 2.5-fold higher HF hospitalisation rate versus white patients.88 In contrast to these findings, a recent report from the ARIC study showed a substantial increase in rates of hospitalisation for acute decompensated HF driven by primarily HFpEF.89 Over 10 years between 2005 and 2014, the average annual percentage increase was +4.3% for black women, +3.7% for black men, +1.9% for white women and +2.6% for white men. In the Olmsted County study, hospitalisation rates did not change significantly during 2000–10.37 An increase in non-CV hospitalisations was paralleled with a decrease in CV hospitalisations, particularly among HFrEF cases. There is a trend toward outpatient treatment of worsening HF, which also likely affects hospitalisation rates for HF over time.90,91
Conclusion
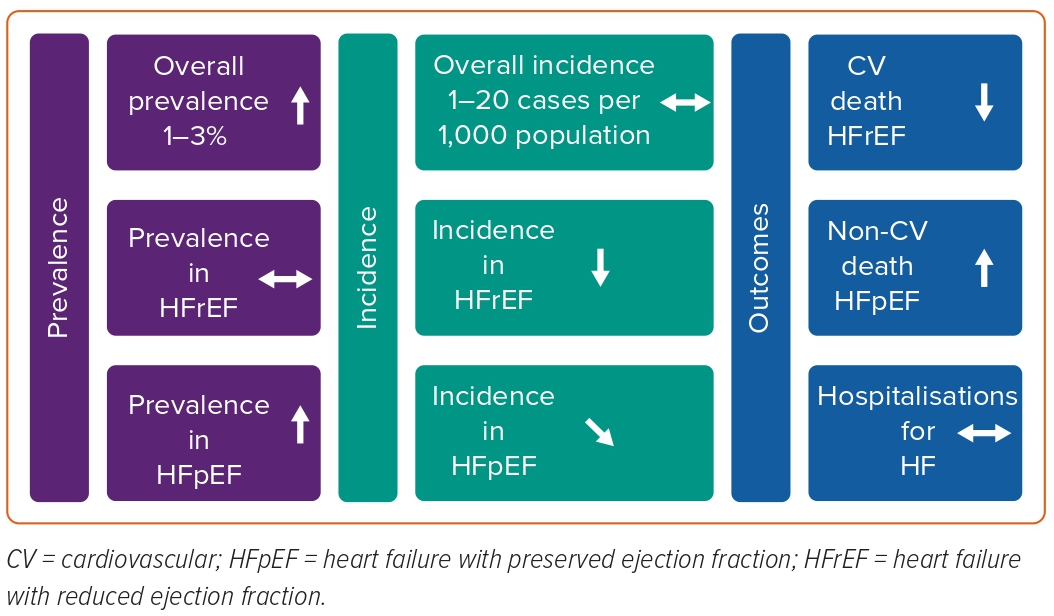
The HF epidemic is changing (Figure 3). Although age-adjusted incidence has stabilised and seems to be declining, the total number of patients living with HF is increasing. Also, the case mix of HF is shifting from HFrEF to a larger proportion of patients with HFpEF, which may become the most common form of HF in the future. HFpEF seems to be distinct from HFrEF, and therapeutic options for HFpEF are only beginning to emerge. HFmrEF has been increasingly well characterised and appears more like HFrEF than HFpEF and medications effective in HFrEF may also be effective in HFmrEF, although this requires further study. Over the past decades, prognosis of HF has slightly improved, but mortality and hospitalisation rates remain high, and many patients progress to advanced HF with few treatment options. CV death is still the major underlying cause of death in HF. However, CV death has been decreasing over time while non-CV deaths have been increasing, particularly in HFpEF. Lastly, very little is known about HF epidemiology in countries outside Europe and North America, but scarce literature suggests the prevalence of HF is rapidly increasing in these regions and that HF is more often prevalent in the young.