Heart failure (HF) is one of the most common reasons for admission to hospital. It is associated with long in-patient stays, and has a high in-hospital and post-discharge morbidity and mortality, whether left ventricular ejection fraction (LVEF) is reduced (HFREF) or normal (HeFNEF).1,2 Congestion, or fluid overload, is a classic clinical feature of patients presenting with HF. In some patients, pulmonary congestion develops very rapidly because of a sudden increase in LV filling pressures, and a precipitating factor is often recognised, such as acute myocardial ischaemia, or uncontrolled hypertension. In this circumstance, the oedema is localised predominantly to the pulmonary airspaces (pulmonary oedema), while the total amount of fluid in the cardiovascular system remains unchanged.3 For most patients, however, congestion is a more generalised process that usually develops more gradually (peripheral oedema), and its management will be the focus of discussion in this review.
Chronic fluid accumulation is responsible for a substantial number of hospital admissions, and identifies patients with a worse prognosis than those admitted due to a sudden increase in LV filling pressures.4 Peripheral congestion in patients with heart failure usually develops over weeks or even months, and patients may present ‘acutely’ having gained over 20 litres of excess fluid, and hence over 20 kg of excess weight. The aim of management is to remove the excess fluid, so that the patient is no longer congested when they leave hospital, now transitioning to a diagnosis of ‘chronic HF (CHF)’. However, for many patients, some degree of congestion remains even with treatment,5,6 and it is not clear how many patients with CHF have subclinical congestion – that is, have an excess of body fluid falling short of the volume required to cause overt peripheral oedema.
Why Do Patients with Heart Failure Retain Fluid?
The development of peripheral oedema in patients with HF is related to fluid excess. As the heart starts to fail, renal perfusion falls. The kidneys respond by increasing the production of renin, leading to more aldosterone production, which is consequently followed by sodium and water retention.7 Arginine vasopressin (AVP) is also released,8,9 further enhancing fluid retention and stimulating thirst. The activation of the renin–angiotensin–aldosterone and AVP systems maintain cardiac preload (more fluids) and afterload (vasoconstriction, mainly due to angiotensin II), thereby maintaining the homeostasis of the cardiovascular system but at a cost of increased systemic venous pressure (VP). The heart itself tends to worsen with time as the failing LV tends to dilate, as does the left atrium, particularly if mitral regurgitation develops. The elevated VP can further reduce renal blood flow as the gradient between mean renal arterial pressure (often itself decreased by the HF process) and VP declines. Glomerular filtration rate falls, enhancing and perpetuating the vicious cycle.10
How Do We Identify Congestion?
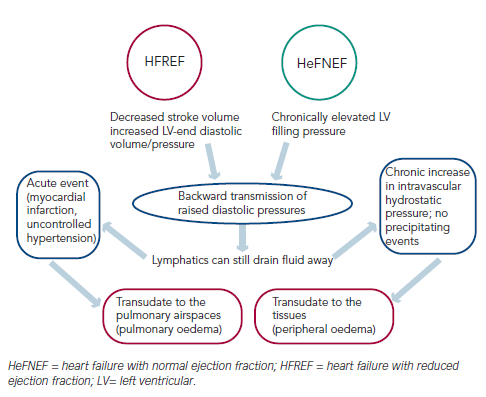
The accumulation of fluids is a gradual process. In normal circulation, there is continuous filtration of fluid from the intravascular space into the tissues at a rate dependent on the gradient between the intravascular and extravascular hydrostatic pressure. Any filtered fluid is then drained by the lymphatics. Overt cardiogenic peripheral oedema develops because the fluid retention results in an increase in intravascular hydrostatic pressure and a commensurate increase in the filtration rate, which eventually exceeds the capacity of the lymphatics to drain fluid away (see Figure 1).
Some patients do not present until they have developed widespread peripheral oedema. In such cases the need for medical intervention is obvious. However, a substantial number of cases of subclinical congestion will not be clinically recognised, despite the presence of symptoms (i.e. breathlessness). In patients with no known cardiac disease, particularly in older people,11,12 the identification of subclinical congestion (and underlying cardiac dysfunction) at an earlier stage might change the trajectory of the disease. In patients who are already known to have HF, whether subclinical congestion is important is not clear.
It used to be said that assessment by an experienced clinician is probably adequate to determine fluid status.13 However, the art of clinical examination is declining, partly because of the widespread availability of echocardiography and other functional or biochemical tests, partly because accurate assessment can take a long time, particularly in patients with poor mobility, and partly because clinical signs are not often specific for the disease; all of this leads to doctors being less skilled in clinical assessment.14 Moreover, clinicians often disagree when faced with typical signs of HF, which may have an unreliable relationship to diagnostic findings, including chest X-rays.15 Nevertheless, a well-conducted clinical examination in patients with suspected HF is still a powerful tool to identify sicker patients who have a worse prognosis, irrespective of their LVEF.16
The most reliable clinical sign indicating volume overload is a raised jugular venous pressure (JVP), which also provides powerful prognostic information.17 However, the clinical assessment of the JVP is often challenging and subjective18 and its assessment by ultrasound might thus be useful. In the vast majority of cases, assessing the jugular vein by ultrasound is possible and allows the identification of patients with more advanced congestion and higher natriuretic peptides (NPs),19 who are at higher risk of adverse outcomes.20 Assessing the inferior vena cava diameter by echocardiography provides complementary information to clinical examination, is validated against invasively measured haemodynamics and is readily available in echocardiographic departments.21,22
The use of NPs as a measure of cardiac dysfunction is advised by current guidelines. NPs are one of the body’s defences against congestion.23 Any stretch of the myocardium leads to an increase in NP level, and raised levels in a treated patient suggests that there is residual congestion, regardless of LVEF. It is important to remember that circulating plasma levels should be adjusted for age and that co-morbidities, including atrial fibrillation, renal dysfunction and obesity, may influence NP levels.24 In clinical settings without easy access to echocardiography, measuring the NP level is a simple and reliable tool, efficient in terms of patient and staff time. Serial NP assessment at home is feasible with a finger-stick test and this approach in high-risk patients might detect possible decompensation early.25
Invasive devices have potential as tools to predict congestion.26 Possible variables that can be measured include trans-thoracic impedance, pulmonary artery pressure and left atrial (LA) pressure. Implantable devices, such as cardiac resynchronisation therapy (CRT) pacemakers and/or defibrillators (ICDs) can measure intrathoracic impedance, thus estimating pulmonary congestion, but their predictive value is still uncertain.27
In the HOMEOSTASIS trial, measurements of LA pressure were taken twice daily in 40 patients with advanced HF. After the first 3 months, treatment was personalised based on the readings, which led to a fall in LA pressure (17.6 mmHg in the first 3 months to 14.8 mmHg; p=0.003), the prescription of higher doses of angiotensin-converting-enzyme inhibitors (ACE-I) and beta-blockers, a lesser need for high doses of loop diuretics and improvement in both New York Heart Association (NYHA) class and LVEF.28
In the CHAMPION trial, pulmonary arterial pressure was measured daily using wireless devices permanently implanted into the pulmonary artery. There was a 37 % decrease in the number of hospitalisations for HF over the 15 months of follow-up.29
An objective measure of ‘congestion’ might be helpful not only to allow physicians to tailor treatments to specific targets, but also to allow more reproducible randomised controlled trials designed to assess decongestive strategies, particularly when markers of cardiac dysfunction (such as LVEF) are not significantly impaired. For example, the ALDO-DHF and TOPCAT trials in patients with HeFNEF suggested that while spironolactone might worsen renal function and anaemia in patients with low circulating NPs (and thus, presumably, little congestion), it significantly reduced HF hospitalisations in patients with higher N-terminal of the prohormone brain natriuretic peptide (NT-proBNP).30,31 The majority of HF trials nowadays include raised NPs as a major criterion for enrolment.
Why Does Congestion Matter?
Congestion is an important cause of symptoms in patients with HF. The discomfort of swollen legs and ascites precipitates hospitalisation. Congestion is associated with the sensation of breathlessness, particularly when patients develop pulmonary oedema and pleural effusions. Congestion reduces hepatic function, and the congested liver can itself be a source of discomfort. As described above, congestion causes renal dysfunction by reducing the transrenal pressure gradient. Anaemia, which is highly prevalent among HF patients, can be made worse by congestion through dilution, and can further exacerbate symptoms and cardiac dysfunction.32
The commonest cause for hospitalisation in patients with CHF is fluid retention and congestion.4 Hospitalisation itself is associated with an adverse prognosis, and repeated hospitalisations are associated with increasingly poor survival.33 Congestion itself, and not just reduced cardiac function, thus appears to be associated with a poor prognosis.
Congestion is a powerful marker of an adverse prognosis and it is thus potentially an important therapeutic target.
How Do We Treat Congestion in Chronic Heart Failure Patients?
Inducing a Diuresis
Diuretics are the mainstay of management for patients with congestion. It has become a truism to state that their use is based on empirical judgement and subjective clinical evaluation, rather than evidencebased medicine.
Different classes of diuretics are used in patients with chronic HF, although loop diuretics (furosemide, bumetanide and torasemide) are the most widely prescribed. They exert their effect primarily by inhibiting the sodium–potassium–chloride co-transporter in the thick ascending limb of the Loop of Henle, by preventing the re-absorption of these ions, a subsequent diuresis occurs. The loop diuretics mediate their effect from the luminal side of the tubule, and so some glomerular filtration is essential to allow them to work.
The beneficial effects of a loop diuretic on JVP, pulmonary congestion, peripheral oedema and body weight have been known for years; diuretics also improve cardiac function, symptoms, and exercise tolerance in patients with HF.34–36 However, no randomised prospective study has ever evaluated their impact on the outcome of chronic HF patients. Particularly in patients with severe renal dysfunction, a reduced response to them is frequently observed and their use alone may be insufficient.
For those responding poorly to a loop diuretic alone, the combination with a thiazide (or thiazide-like) diuretic can be very potent. Although metolazone is often used in this scenario, there is little evidence that it is superior to other agents, such as bendroflumethiazide.37 The trial experience of combining several classes of diuretics is still limited to just above 300 patients enrolled in small, mechanistic studies.38
Mineralocorticoid receptor antagonists (MRAs) are, of course, also diuretics. Two large trials39,40 have shown that adding spironolactone or eplerenone to standard treatment in symptomatic patients with reduced LVEF (either chronically or after a recent myocardial infarction) produces morbidity and mortality benefits. Whether the beneficial effects are due to a reduction in congestion is not at all clear given the wide range of actions of MRAs.41 The dose of MRA used to induce a diuresis is typically much higher than that used to treat chronic HF.
The clinical benefits observed following the introduction of loop diuretics are counterbalanced by a more marked activation of the renin– angiotensin system.42 An important matter to be considered is thus whether adding a diuretic in patients who are not clinically congested has any benefit. It is not at all clear whether diuretics lead to improved exercise capacity and/or improvement in biochemical measures of subclinical congestion (including NPs). Francis and colleagues showed that the acute injection of a loop diuretic (furosemide 1.3 ± 0.6 standard deviation [SD] mg/kg body weight) in patients who are not congested can provoke transient adverse haemodynamic effects, with an increase in LV filling pressures and a fall in stroke volume index,43 with restoration of better haemodynamics and neurohumoral variables only after several hours. Other reports suggest that using diuretics unnecessarily (when there is no evidence of congestion) for a longer period of time might decrease systolic and diastolic blood pressure and increase circulating levels of renin compared with placebo.44
Retrospective studies have raised concerns about a possible detrimental effect of the long-term use of loop diuretics in HF patients, possibly caused by chronic and sustained adverse neuroendocrine activation.45,46 However, it is also logical to think that patients with more severe HF will be prescribed more loop diuretics, which would have then been associated with the adverse outcome.47 The relation between diuretic dose and outcome needs more clarification, but there is the general belief that achieving the lowest tolerated dose, or even a definite withdrawal from loop diuretics, might be beneficial.
A small number of studies have attempted to identify patients who might be able to tolerate diuretic withdrawal. Perhaps a third of patients with HF can tolerate loop diuretic withdrawal, particularly if LVEF is above 27 % or the baseline dose of loop diuretics is ≤40 mg furosemide/ day;48 this proportion significantly increases in patients who are not clinically considered at high risk of deterioration49 and might reach up to 90 % success at 3 months follow-up when the LVEF is normal.50
Vaptans
Patients with HF have raised vasopressin (AVP), which causes water re-absorption in the collecting ducts of the nephrons. Vaptans block the action of vasopressin on its receptors, thus leading to loss of water alone without a natriuresis – a so-called aquaresis. In 142 patients with severe HF symptoms, and compared with placebo, a single intravenous dose of conivaptan (20 or 40 mg) significantly reduced pulmonary capillary wedge pressure and right atrial pressure during the first hours following administration, also increasing urine output at a dose-dependent amount.51 However, in the EVEREST trial,52 the use of tolvaptan was associated with no change in clinical outcomes in a population of over 4,000 patients who were admitted with acute HF. Enthusiasm for the routine use of vaptans has thus waned, but they could certainly be helpful in patients who have hyponatraemia. Gheorghiade and colleagues showed that tolvaptan at different doses (30, 45 or 60 mg/day) for 25 days was effective in decreasing oedema, and also normalised serum sodium in hyponatremic patients compared with placebo.53
It is possible that vaptans might be better than a loop diuretic as standard care. In a recent study, 83 patients treated on optimal medical treatment, with severe HF and clinical congestion, were randomised to placebo, monotherapy with tolvaptan 30 mg/day or furosemide 80 mg or both tolvaptan 30 mg and furosemide 80 mg once daily for 7 days after a 2-day run-in period of low-sodium diet (2 mg/ day). Tolvaptan, or tolvaptan plus furosemide, were well tolerated and produced a similar increase in urine output, greater than furosemide or placebo, without affecting blood pressure or other electrolytes apart from sodium, which increased (although within normal values).54
The role of vaptans in routine practice is still uncertain, but several trials are on the way. At the moment, European Society of Cardiology (ESC) guidelines only recommend that tolvaptan may be used for patients with acute HF and resistant hyponatraemia; in the US, vasopressin antagonists have a class IIb recommendation for the short treatment of acute HF with congestion and persistent severe hyponatremia, at risk of (or having) active cognitive symptoms.
Other Drugs
ACE-inhibitors are the first-line treatment for chronic HF patients with reduced systolic function, unless contraindicated. They have a wide range of effects including the promotion of diuresis and the renal excretion of sodium, principally by blocking the effects of angiotensin II in the kidney and angiotensin II-mediated aldosterone secretion. In turn, ACE-inhibitors reduce the circulating blood volume, and both venous and arterial pressures; moreover, they not only improve the peak oxygen consumption but also decrease NP plasma levels in symptomatic55 or asymptomatic patients.56 The effect of ACE-inhibitors on NPs is independent of co-administration of beta-blockers.57
In a trial that pre-dates modern therapy, patients whose symptoms and congestion were well-controlled were unable to maintain clinical stability for long periods on diuretics alone. The risk of clinical decompensation was decreased when diuretics were combined with digoxin or an ACE-I.58 The addition of a direct renin-inhibitor to an ACE-I might further enhance diuresis and decrease NP levels,59 but whether this translates into an improved long-erm outcome is not known yet; results for the ongoing ATMOSPHERE trial are expected soon.
LCZ 696 combines angiotensin receptor blockade (with valsartan) and inhibition of neprilysin, an enzyme that degrades NPs, with sacubitril. LCZ 696 decreases the risk of death and hospitalisation for HF in patients with stable chronic HF compared with enalapril. The drug increases circulating active NP, as shown by higher urinary levels of cycling guanosine monophosphate (GMP), the NP’s second messenger, suggesting that it may have some role in reducing congestion.60
It has long been known that digoxin used alone in patients with severe congestion – particularly those with atrial fibrillation – can cause a profound diuresis. The effect is presumably secondary to the improvement in haemodynamics induced by both heart rate slowing and by digoxin’s positive inotropic effect, but there does appear to be a modest direct renal effect of digoxin.61 Since the introduction of loop diuretics, digoxin is very rarely used only for its diuretic effects.
Levosimendan causes vasodilation of the coronary arteries and systemic resistance vessels, decreasing preload and afterload. Some recent reports suggest that short, intermittent courses of intravenous levosimendan might decrease NPs and possibly HF hospitalisation.62,63 Larger trials are ongoing, evaluating the efficacy of this novel approach (LAICA: NCT00988806 and ELEVATE NCT01290146).
Other Factors
The long-term education of patients with HF is of fundamental importance, to emphasise medication adherence and monitor symptoms indicating progression of disease. It might be that some patients remain congested just because they do not take their prescribed medications. Around 25 % of patients have difficulties in keeping their follow-up appointments or taking their drugs,64 and this proportion increases over time after diagnosis.65 A substantial proportion of patients continue to smoke despite the adverse diagnosis.64 Nurses, physicians and other members of a multidisciplinary team, including a pharmacist,66 can provide education to patients, increase their compliance and, more importantly, improve quality of life, decrease readmissions rate and alleviate the economic burden of this increasingly common disease.67
During times of severe fluid retention, simple interventions, such as continuous bed rest, might enhance diuresis and significantly reduce body weight compared with bed rest during night only;68 also diurnal postural changes might influence the diuretic action, which is enhanced by supine position compared to the erect.69 Although fluid restriction is an intervention mentioned by current guidelines for patients with HF, a recent meta-analysis including five studies suggests this therapy has no benefit compared with liberal fluid intake on mortality, admission or thirst in patients with HF.70
The role of sodium restriction is not clear, although part of the traditional management of HF and recommended in guidelines (albeit with an acknowledged low grade of evidence to support the recommendations).71 In acute HF and congestion, the only effect of sodium restriction appears to be to increase the sensation of thirst.72 In patients with chronic HF, a normal sodium diet is associated with better outcomes, albeit on the background of very high loop diuretic dose.73 It seems sensible to suggest to patients that they should not add large quantities of salt to their diet, but excessive restriction has no role.
Newer technologies, such as home-telemonitoring, can be used to educate patients further but also to allow health care professionals to monitor patients’ symptoms and physiological variables. Early studies of telemonitoring suggested that it was helpful, but more recent studies are less convincing, perhaps because the ‘standard care’ limb of trials has improved markedly.74
Treating Congestion in Heart Failure and Normal Ejection Fraction
In patients with HF and HeFNEF, there is no clear evidence that ACEinhibitors affect NP levels.75 In a study that enrolled 150 patients with HeFNEF randomised to diuretics alone (either furosemide or thiazides, depending on the level of congestion) or diuretics plus irbesartan or ramipril, all treatments improved quality of life quickly (after 12 weeks). Plasma NT-proBNP fell after 12 months in the three groups, but the fall only reached statistical significance in the irbesartan (–124 (SD 302) pg/ml; p=0.01) and ramipril (-173 (SD 415) pg/ml; p=0.03) groups, suggesting a synergistic effect between the renin–angiotensin system inhibitors and diuretics.76
In patients with HeFNEF, and compared with valsartan, LCZ 696 was effective in reducing NT-proBNP after 12 weeks of treatment in the PARAMOUNT trial although the difference between treatment groups was no longer significant after 36 weeks of follow-up.77 Whether this translates into outcome benefits is currently under investigation in the large PARAGON trial, which should complete in 2019.
Ultrafiltration and Home Abdominal Paracentesis
In the most severe cases of HF, renal dysfunction and diuretic resistance often occur, and limit the available therapeutic resources to decrease congestion. While ultrafiltration is an invasive solution usually reserved for patients with severe acute HF, peritoneal dialysis (PD) is a home-based, intermittent, therapeutic option in which the removal of the excess fluid takes place using the peritoneum as a filter. Two recent studies of patients with advanced HF complicated by renal failure have reported that PD is feasible, and that it might decrease body weight, and improve symptoms and functional status.78,79 A recent systematic review of 21 studies (n=673 patients) suggests that PD improves ventricular function and decreases the number of days spent in hospital with little risk of peritonitis (14 %/year).80
Conclusions
Congestion is a cardinal clinical feature of chronic HF, and is linked to adverse outcomes. Although several interventions might improve congestion, it often remains underdiagnosed. Little is known about the effects of the anti-congestive drugs par excellence, the diuretics, on hard outcome measures, such as mortality. More objective measures of congestion (such as raised NPs, or dilated vena cava on ultrasound) might allow the identification of higher risk patients: trials are then needed to test therapeutic decongestive strategies in order to establish whether increased loop diuretic doses, combined diuretics or more aggressive interventions, such as peritoneal dialysis, might improve the patient’s clinical status and, possibly, outcome.








