The global burden of heart failure (HF) is increasing with each passing decade and has become a major challenge for cardiovascular (CV) disease management systems. Over the last three decades, there have been rapid advances in targeted pharmacotherapy to improve the prognosis of HF in terms of reducing mortality and hospitalisation. Multiple landmark clinical studies have established the roles of drugs, such as β-blockers, renin–angiotensin–aldosterone system (RAAS) blockers and sodium–glucose cotransporter 2 inhibitors (SGLT2I), in the management HF. One of the first drugs shown to improve HF prognosis was spironolactone, the prototype mineralocorticoid receptor antagonist (MRA). To date, it remains one of the most well-studied, least expensive, and has one of the highest potentials for mortality reduction among all the medications used in HF management. This review evaluates the role of aldosterone antagonism across the spectrum of HF.
Role of Aldosterone in Heart Failure and Cardiovascular Disease
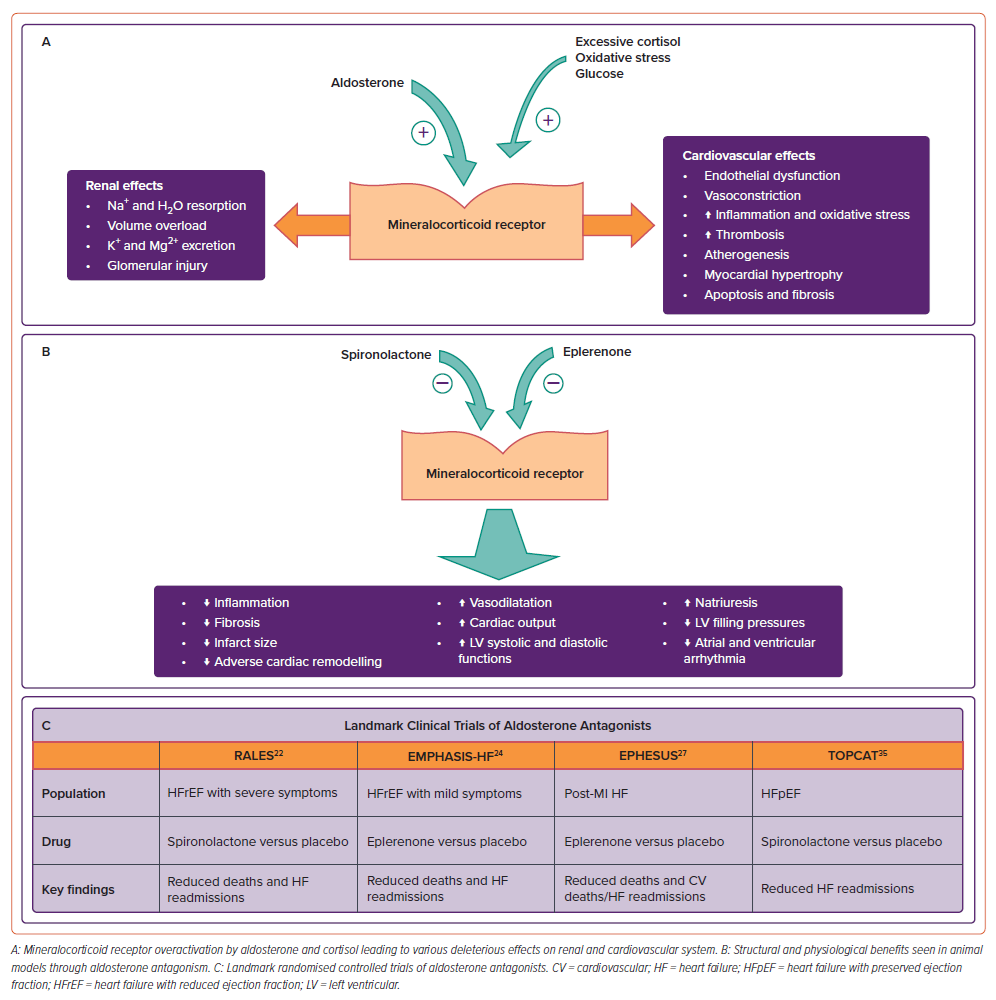
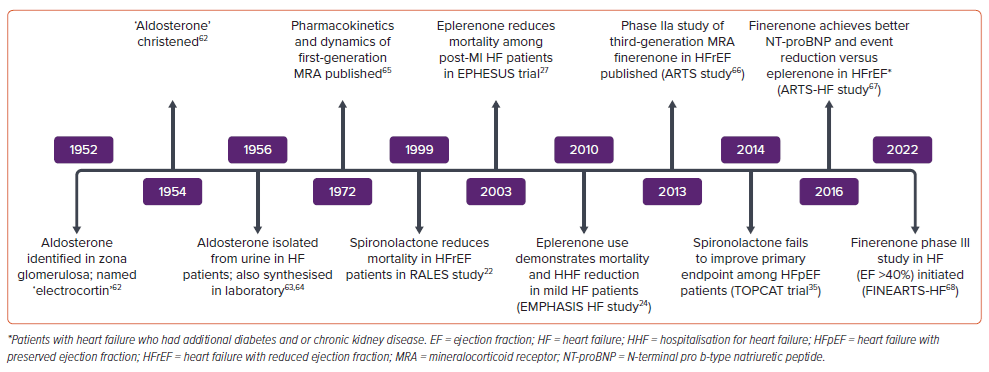
Aldosterone, a well-known neurohormone, was discovered almost seven decades ago as a key factor responsible for water and electrolyte homeostasis. Its role is now well-established in the pathophysiological progression of HF. Low cardiac output leading to renal hypoperfusion acts as a stimulant for the overactivation of RAAS. This cascade, which is initiated with the increased secretion of renin from the juxtaglomerular cells of the kidney, through angiotensinogen, angiotensin I and II in the liver and via AT1 receptors, ultimately culminates in increased secretion of aldosterone from the zona glomerulosa of the adrenal cortex. Increased aldosterone in turn activates mineralocorticoid receptors (MR) in renal tubular cells and leads to sodium and water retention, along with potassium and magnesium excretion. This increase in intra-vascular and extra-vascular volume is central to the progression of HF. Several extra-adrenal sources of aldosterone have been identified, including the brain, adipocytes, smooth muscle cells, myocardium and blood vessels.
Apart from the kidneys, MRs are also expressed in the heart and blood vessels.1 These receptors are responsible for extra-renal physiological and pathological functions of aldosterone, especially adverse CV effects. Increased aldosterone levels lead to endothelial dysfunction and deranged vessel reactivity. This is due to the activation of pro-inflammatory cytokines leading to increased oxidative stress, reduced nitric oxide availability and reduction in vascular antioxidant capacity.2–4 By activating plasminogen activator inhibitor-1 aldosterone promotes thrombosis and extracellular collagen deposition.5 All these contribute to the pro-atherogenicity of aldosterone. Aldosterone also causes sympathetic activation, decreased baroreceptor sensitivity, apoptosis, myocardial fibrosis, hypokalaemia and hypomagnesaemia.6 Thus, MR inhibition can help ameliorate these deleterious effects of aldosterone, thereby preventing hypertension, left ventricular (LV) dysfunction, HF and life-threatening arrhythmia. (Figure 1)
The combination of both renal and extra-renal effects of aldosterone antagonism makes for an attractive pharmacological target in the management of various stages of HF, both for symptomatic and prognostic benefits. This has led to clinical trials in the last three decades to evaluate the role of aldosterone antagonism in a variety of indications, including HF with reduced ejection fraction (HFrEF), post-MI HF and HF with preserved ejection fraction (HFpEF; Figures 1 and 2).
Aldosterone Antagonism in Heart Failure
Neurohormonal activation (especially RAAS) is central to the pathophysiology of HF. Angiotensin-converting enzyme inhibitors (ACEI) and angiotensin-2 receptor blockers (ARB) are the predominant classes of drugs available for inhibition of RAAS, but this inhibition is far from perfect. Due to a phenomenon called ‘aldosterone escape or aldosterone breakthrough’, aldosterone levels return to normal or may even increase within few months of ACEI therapy.7 This escape can mitigate the beneficial effects of both ACEI and ARB therapy. Cortisol is another major MR agonist in patients with HF and is responsible for inflammation and fibrosis.8 Hence, complete suppression of RAAS requires additional specific inhibition at aldosterone receptor level. Aldosterone antagonists (spironolactone and eplerenone) by acting at the aldosterone receptor level, in combination with ACEI/ARBs, lead to near-complete suppression of RAAS.
Structural and Mechanistic Evidence of Aldosterone Antagonism in Heart Failure
Animal experiments have demonstrated the efficacy of MRAs in conjunction with ACE inhibition, leading to significant natriuresis and lowering of LV filling pressures, improved LV systolic and diastolic functions and cardiac output.9–11 In rats with MI, treatment with MRAs resulted in better infarct neovascularisation and reduced infarct size, LV thinning and dilatation.11,12 MRAs have been shown to have nitric oxide-mediated vasodilatory properties as well as intrinsic inotropic action.13,14
There is a reduction in pro-inflammatory cytokines,15 reversal of collagen metabolism, e.g. reduction in pro-collagen type III amino-terminal peptides and plasma level of metalloproteinases16,17 This effect was consistent in ischaemic as well as non-ischaemic hearts. All these in turn lead to reduced or delayed myocardial fibrosis.18 MR inhibition also reduces atrial and ventricular arrhythmia.19,20
Combined, the above-mentioned beneficial actions form the foundation of the pathophysiological mechanism for positive clinical outcomes associated with aldosterone antagonism in patients with HF.
Clinical Trials in Heart Failure with Reduced Ejection Fraction
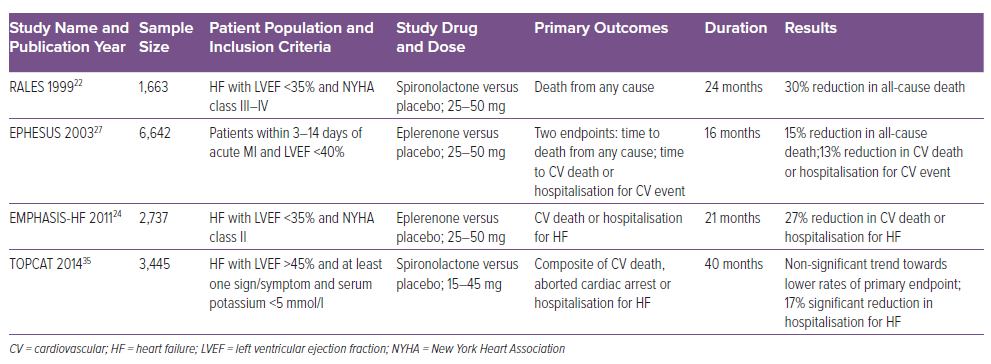
Two major randomised controlled trials (RCTs), RALES and EMPHASIS-HF, evaluated the effect of MRAs in HFrEF (Table 1).
As a prelude to the RALES trial, a RALES dose-ranging study, comparing four doses of spironolactone (12.5, 25, 50 and 75 mg/day) against a placebo along with standard care of the time, was conducted in symptomatic New York Heart Association (NYHA) class II–IV congestive HF patients.21 Though the dose of 75 mg/day was more efficacious in terms of reducing N-terminal pro-atrial natriuretic factor (pro-ANF), it led to a significant increase in the incidence of hyperkalaemia compared to placebo (24% versus 5%). Hence, a dose of 25 mg of spironolactone was chosen for the RALES trial to maximise adherence to the study drug while minimising adverse events.
The RALES trial was published in 1999 and established the role of aldosterone antagonists in the management of HF.22 It was a placebo-controlled (spironolactone 25 mg/day versus placebo) double-blind trial and included 1,663 patients with severe HF. Patients had to be in NYHA class IV within 6 months before randomisation or in NYHA III or IV at the time of randomisation and additionally have a LV ejection fraction (LVEF) ≤35%. Patients were excluded if they had serum creatinine >2.5 mg/dl or serum potassium >5.0 mEq/l. Most patients were already being prescribed loop diuretics (100%), ACEI (95%) and digitalis (75%). Only 11% of patients were taking β-blockers. It is important to note that β-blockers were not established as a standard HF medication at that time. The study was prematurely discontinued at a mean follow-up of 24 months because an interim analysis revealed positive outcomes. The primary endpoint, all-cause mortality, was reduced significantly by 30% (RR 0.70; 95% CI [0.60–0.82]; p<0.001) and this was driven by a reduction in both sudden cardiac death (SCD) and death due to HF hospitalisations. Hospitalisations for worsening HF were also reduced by 35% (RR 0.65; 95% CI [0.54–0.77]; p<0.001). There was also a significant improvement in symptoms of HF (NYHA class) as well as brain natriuretic peptides (BNP) levels. Subgroup analyses revealed that the mortality benefits of spironolactone were greater in patients who were already taking ACEI and β-blockers. The likely reason is that spironolactone in combination with these two classes of drugs causes a higher degree of aldosterone suppression. Gynaecomastia or mastodynia was observed in as many as 10% of patients, while severe hyperkalaemia was seen only in four patients receiving spironolactone.
It must be emphasised that the dosage of spironolactone was non-diuretic hence the mortality benefits observed in the RALES trial most likely stemmed from anti-inflammatory and anti-fibrotic effects of aldosterone antagonists as mentioned above.
A substudy of RALES observed that spironolactone-associated mortality benefits were significant only in patients with higher baseline levels of pro-collagen type III amino-terminal peptide. This study highlights one of the putative extra-renal mechanisms of aldosterone antagonists, thereby benefiting HF patients.23
The other large RCT to evaluate aldosterone antagonists in chronic HFrEF patients is EMPHASIS-HF.24 This trial differs from RALES in several aspects. This study used eplerenone, a highly selective MRA, and aimed to include patients with mild HF. It enrolled 2,737 patients with chronic HF (LVEF ≤30%) with mild symptoms (NYHA class II) and a history of hospitalisation within 6 months or elevated BNP. The major exclusion criteria were acute MI, severe HF (NYHA class III and IV), serum potassium ≥5 mEq/l, estimated glomerular filtration rate (eGFR) ≤30 ml/min/1.73 m2. Baseline HF therapy included diuretics (86%), ACEI or ARB or both (93%), β-blockers (87%) and digitalis (28%). The study was terminated early after a median follow-up of 21 months. There was a 37% reduction in composite endpoints of CV mortality or HF hospitalisations (HR 0.63; 95% CI [0.54–0.74]; p<0.001). There was also 24% reduction in all-cause mortality, 42% reduction in all-cause hospitalisation and 23% reduction in hospitalisations due to HF. The number needed to treat (NNT) to prevent one primary outcome event was 19 patients per year, while the NNT to prevent one death was 51 patients per year.
Serum potassium ≥5.5 mEq/l was seen in 11.8%, while severe hyperkalaemia (serum potassium ≥6 mEq/l) was seen only in 2.5% of patients in the eplerenone group. Gynaecomastia and other breast disorders were rare (0.7%) in the eplerenone arm. There was no increased risk of hospitalisation due to worsening renal functions or hyperkalaemia in the eplerenone group as compared to placebo. As per a later pre-specified subgroup analysis, though the risk of these complications was higher in certain groups (>75 years of age, diabetes, history of chronic kidney disease, lower systolic blood pressure), net survival benefit was observed even in these high-risk groups.25
Another pre-specified analysis of the EMPHASIS-HF trial revealed a significantly reduced incidence of new-onset AF or flutter in patients of HF treated with eplerenone as compared to placebo (2.7% versus 4.5%; HR 0.58; 95% CI [0.35–0.96]; p=0.034).26
Aldosterone Antagonism in Post-MI Heart Failure
The EPHESUS trial evaluated the effect of eplerenone in patients with acute MI complicated by LV systolic dysfunction with HF or diabetes.27 A total of 6,642 acute MI (3–14 days after the index event) patients with LVEF ≤40% and HF were included in the trial. For patients with diabetes and LV dysfunction, criteria of HF symptoms were not needed, as they were considered to have equivalent risk to those with HF. Ninety per cent of patients had symptoms of HF, while 32% of patients had diabetes. Patients with serum potassium ≥5 mEq/l and serum creatinine ≥2.5 mg/dl were excluded. Concomitant HF therapy included ACEI/ARB in 87% and β-blockers in 75% of patients. After a mean follow-up period of 16 months, patients in the eplerenone arm showed a significant reduction in two pre-defined endpoints of all-cause mortality (HR 0.85; 95% CI [0.75–0.96]; p=0.008) and combined endpoints of CV deaths and HF hospitalisation (HR 0.87; 95% CI [0.79–0.95]; p=0.002). Among the secondary endpoints, SCD also was significantly reduced (HR 0.79; 95% CI [0.64–0.97]; p=0.03). There was no excess of sex-related side-effects as expected with higher MR selectivity. Severe hyperkalaemia was significantly increased with eplerenone (5.5% versus 3.9%; p=0.002). Interestingly, the incidence of hypokalaemia, which is a known factor driving sudden cardiac deaths, was significantly lower with eplerenone (8.4% versus 13.1%; p<0.001).
A post hoc analysis of EPHESUS data revealed that mortality reduction by eplerenone was most robust when it was started early after acute MI (3–7 days post MI) rather than later (beyond 7 days post MI).28 Another analysis of the same data at 30 days revealed a significant reduction in all-cause mortality, driven primarily by a reduction in sudden deaths.29 Both these analyses emphasise the importance of early initiation of aldosterone antagonists after acute MI with LV dysfunction or diabetes.
Some studies have attempted to evaluate aldosterone antagonists early after acute MI without HF. In the REMINDER trial, eplerenone within the first hour after acute MI led to a reduction in primary composite endpoints but it was solely driven by higher BNP/NT-proBNP levels in the placebo arm.30 Another randomised study, the ALBATROSS trial (IV potassium canrenoate followed by oral spironolactone) failed to show mortality benefit in early acute MI patients.31 A meta-analysis by Dahal et al. that included 10 randomised trials (including the REMINDER and ALBATROSS studies) and 4,147 patients, showed a lower risk of mortality with MRA in patients with ST-elevation MI (STEMI) without HF or LVEF >40% (2.4% versus 3.9%; OR 0.62; 95% CI [0.42–0.91]; p=0.01).32 A recent MINIMIZE-STEMI trial failed to show infarct size reduction in STEMI patients when MRA (IV bolus of potassium canrenoate followed by oral spironolactone for 3 months) was initiated before reperfusion.33
In the light of contemporary evidence, mortality benefits with early initiation of MRA are robust in acute MI with HF patients only. In patients without HF, MRA may be safe but its effect on mortality is dubious and, hence, not advisable until further evidence is available in the form of adequately powered RCTs.
Reduction of SCD is another striking feature of MRA use in HF. A patient-level meta-analysis of three pivotal MRA studies revealed a 23% reduction in SCD in patients with HF and LV dysfunction. The benefits were higher in younger patients (<65 years) and those on baseline β-blocker therapy.34
Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction
Following the success of MRAs in clinical trials in HFrEF, the investigators began to gather evidence about its efficacy in patients with HFpEF. It was hypothesised that the aldosterone antagonism may be one of the key pathways to target the pathophysiological construct of HFpEF which had been speculative. The absence of any targeted therapy for HFpEF also made these trials a centre of attention for HF physicians. Many clinical studies have been conducted over the last two decades but largely failed to establish a conclusive case for the blanket use of aldosterone antagonism in HFpEF.35–37
Though the largest clinical trial on this subject, the TOPCAT trial failed to show the advantage of spironolactone in reducing the primary composite outcome of death from CV causes, aborted cardiac arrest, or hospitalisation for the management of HF in the overall cohort of HFpEF patients, interesting conclusions can be drawn from the subgroup analysis of this trial.35,38 Subjects enrolled in the US compared to those enrolled in Russia and Georgia had higher event rates in the placebo arm (31.8% versus 8.4%). They also had better response to spironolactone therapy because with a higher event rate, the response to medication was easier to prove. Also, patients in the US were enrolled predominantly from the high BNP stratum compared to those in Russia and Georgia, where randomisation happened predominantly from the hospitalisation stratum. In the hospitalisation stratum, spironolactone did not affect the time to composite outcome (HR 1.01; 95% CI [0.84–1.21]; p=0.92), whereas in the BNP stratum, spironolactone showed a benefit (HR 0.65; 95% CI [0.49–0.87]; p=0.003).
The subgroup analysis of this trial instigated a series of studies to determine clinical and biochemical sub-cohorts in the spectrum of HFpEF patients, who might benefit from specific therapies. Unlike HFrEF where only symptoms plus a cut-off value of ejection fraction was sufficient in deciding specific prognostic therapies, in HFpEF a more complex system of identifying high-risk cohorts was required. Investigators have tried to look at the data from the TOPCAT trial and have identified subgroups with different prognoses and different responses to spironolactone from the overall population of HFpEF.38–40 It has been identified that the P3 phenotype of HFpEF (obesity, elevated inflammatory markers and high renin activity), will have more adverse clinical outcomes. It is this high-risk group of HFpEF patients who will be more likely responders to the targeted therapy with aldosterone antagonists.39
Aldosterone Antagonists in Acute Decompensated Heart Failure
There is scarcity of prospective evidence regarding MRA use in patients of acute decompensated HF. A propensity score-matched analysis of 2,068 Japanese patients from the Kyoto Congestive Heart Failure (KCHF) registry revealed that MRA use at discharge was associated with significant reduction in cumulative HF readmission rates at 1 year (HR 0.70; 95% CI [0.60–0.86]; p<0.001) while no reduction in death due to HF or all-cause mortality could be demonstrated.41 Another study which included a post hoc analysis of the RELAX-AHF-2 study showed that in-hospital initiation of MRA resulted in significantly improved prognosis at 6 months post-discharge in terms of all-cause deaths (HR 0.76; 95% CI [0.60–0.96]; p=0.02), cardiac deaths (HR 0.77; 95% CI [0.59–1.01]; p=0.06), hospitalisation for HF or renal failure (HR 0.72; 95% CI [0.60–0.86]; p=0.0003) and combined endpoints of CV death and/or hospitalisation for HF or renal failure (HR 0.71; 95% CI [0.61–0.83]; p<0.0001).42 Notably, these benefits accrued were independent of LVEF.
Contemporary Guidelines and Recommendations
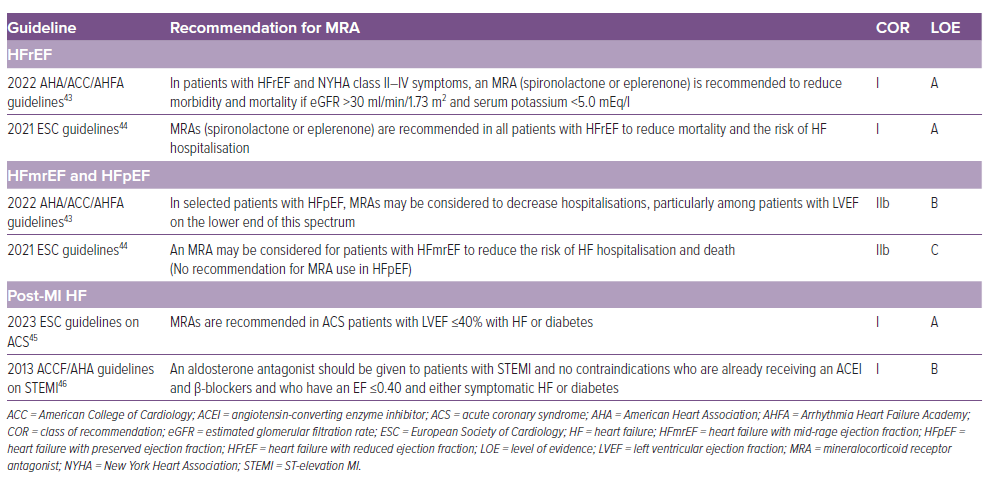
Aldosterone antagonists (spironolactone and eplerenone) have unambiguous favourable evidence in symptomatic patients of HFrEF to reduce mortality and HF hospitalisations and should be prescribed in addition to ACEI or ARB or angiotensin receptor and neprilysin inhibitor (ARNI) and β-blockers (class IA recommendation).43,44 However, evidence in patients with HFmrEF and HFpEF is indirect and based on subgroup analyses of randomised trials designed to address HFrEF and HFpEF. MRAs may be considered in patients with HFmrEF and HFpEF to reduce HF hospitalisations.43,44 Aldosterone antagonists are strongly recommended in patients with acute coronary syndrome who have LV dysfunction (LVEF ≤40%) and HF symptoms or diabetes.45,46 (Table 2)
The starting dose of MRAs (both spironolactone and eplerenone) is 25 mg/day and should be uptitrated gradually to 50 mg/day, if tolerated well, over next 4–8 weeks.47 A lower dose of 12.5 mg/day or 25 mg on alternate day may be the preferred initial dose if eGFR is 30–50 ml/min/1.73 sqm.
Regular follow-up with biochemical monitoring for serum electrolytes and renal function should be done at the first and fourth weeks of drug initiation and every dose escalation, thereafter every 4 weeks and then 3–6 monthly if these parameters are within normal limits. Serum biochemistry must be monitored more frequently in patients with deranged renal function and borderline serum potassium (>5 mEq/l). MRA dose should be reduced to half or alternate day if serum potassium rises >5.5 mEq/l or serum creatinine rises >2.5 mg/dl and close monitoring of above parameters is done. If the patient develops severe hyperkalaemia (serum potassium >6 mEq/l), MRA must be stopped, therapeutic measures should be taken to correct serum potassium and MRAs restarted only when serum potassium is <5 mEq/l.
Barriers to Mineralocorticoid Receptor Antagonist Therapy in Clinical Practice
Despite the irrefutable evidence and guideline recommendations, use of MRA in clinical practice remains low. In a Danish study of HF patients, only 40% of patients were prescribed MRA within 6 months of their HF diagnosis. Unfortunately, over a period of 5 years around 50% of these patients stopped their therapy.48 In a community-based study, renal dysfunction, hypotension and hyperkalaemia were major barriers for not prescribing MRAs.49
Hyperkalaemia remains an important reason for non-prescription as well as underdosing and withdrawal of MRA.50 Patients with hyperkalaemia were 62% less likely to receive MRA in a large nationwide HF clinic survey from Denmark.51 Moreover, the incidence of hyperkalaemia is higher in a real-world scenario than in the controlled environment of an RCT.52 However, it is vital to note that a sub-analysis of the EMPHASIS-HF study showed that development of hyperkalaemia failed to truncate the benefits from eplerenone use.53 Similarly, patients in the EPHESUS study who had elevated potassium levels early after drug initiation had better improvement in outcomes.54 Hence, these data underscore the fact that every attempt should be made in practice for abidance of MRA therapy and pre-empting the factors associated with MRA withdrawal.
The first and foremost method for improving adherence is regular monitoring of renal function and potassium. Unfortunately, despite adequate emphasis by guidelines, the monitoring rates remain low in clinical practice. Secondly, careful attention should be paid to subsets of patients predisposed to hyperkalaemia. In an analysis from the RALES study, advancing age, higher NYHA class, prior diabetes, low eGFR, baseline potassium levels, background ACEI use, background β-blocker use, and drug dose were predictors of hyperkalaemia.55 These patients should undergo more frequent monitoring for renal function and hyperkalaemia than recommended above. MRA should be avoided in patients with baseline serum potassium ≥5 mEq/l, serum creatinine ≥2.5 mg/dl or eGFR ≤30 ml/min/1.73 sqm. Guidelines also recommend using potassium binders (e.g. patiromer and sodium zirconium cyclosilicate) to facilitate use of MRAs in patients with elevated serum potassium (≥5.5 mEg/L) and those with tendency to recurrent hyperkalaemia with MRA therapy.41,42,56,57 Concomitant ARNI and SGLT2I use has been shown to reduce incidence of MRA-associated hyperkalaemia and patients were less likely to discontinue therapy with MRAs.58,59
Any episode of hyperkalaemia should initiate a meticulous search for underlying causes, including significant drug-drug interaction. Concomitant usage of other potassium-raising drugs with MRAs should be avoided, e.g. nonsteroidal anti-inflammatory drugs, potassium-sparing diuretics, potassium salts and supplements, antibiotics (IV penicillin G potassium and pentamidine), tacrolimus and cyclosporin. In addition, CYP3A4 inhibitors (e.g. ketoconazole, erythromycin and grapefruit juice) may also significantly raise serum levels of eplerenone, thereby leading to an increased tendency of hyperkalaemia. If the use of these drugs remains inevitable, the dose should be reduced to avoid severe hyperkalaemia.60 Patient education, avoiding a potassium-rich diet, and multidisciplinary collaboration among the treating cardiologist, nephrologist and dietician play a crucial role in managing this fine balance and avoiding treatment interruption.
Gynaecomastia, mastodynia and menstrual irregularities with spironolactone can be disabling for some patients. Eplerenone is a highly selective aldosterone antagonist that is essentially devoid of these adverse effects and can be a suitable alternative.
The Future
Spironolactone and eplerenone significantly lower mortality in patients with HFrEF but, due to fear of hyperkalaemia and hormonal side-effects, remain under-prescribed. Newer non-steroidal MRAs (e.g. finerenone) are emerging as an attractive alternative due to their high specificity and affinity to MRs and have only minimal risk of hyperkalaemia and sexual adverse effects. Finerenone has already proven its efficacy and safety in patients with diabetic nephropathy and are discussed further in a separate segment of this issue.61 As opposed to its predecessors, which were dreaded for their risk of hyperkalaemia and renal adverse effects, finerenone has emerged as cardio-renal protective therapy. However, its utility as an HF treatment is yet to be established in large RCTs. The FINEARTS-HF study (NCT04435626) is evaluating the utility of the drug in >6,000 patients with HF (LVEF>40%). The FINALITY-HF (NCT06033950) is using finerenone in HFrEF patients who were intolerant to MRAs or not prescribed this class of drugs due to contraindications. It is a clinical endpoint-oriented study with time to CV death or HF event as the primary endpoint. Moreover, it also needs to be assessed as a part of combination therapies, e.g. finerenone in combination with SGLT2I. The MIRACLE study (NCT04595370) is examining the role of the combination therapy with SGLT2I in HF with CKD. Another non-steroidal MRA, esaxerenone, is also in the pipeline. It has been shown to be effective in lowering blood pressure in hypertensive patients and offer reno-protection in patients with diabetic kidney disease.
Inhibition of production of aldosterone via inhibition of aldosterone synthase enzyme offers an alternative therapeutic avenue for direct aldosterone antagonism compared to MRA. These drugs have the additional advantage of blocking the non-genomic effects of aldosterone which remains unthwarted with MRA therapy. Baxdrostat is the prototype highly selective and potent aldosterone synthase inhibitor, which is currently undergoing phase III clinical trials for resistant hypertension.
Conclusion
MRAs are not just any potassium-sparing diuretics; robust clinical trials have shown that spironolactone and eplerenone prevent deaths as well as HF readmissions in patients with HFrEF. The impact in patients with HFmrEF and HFpEF is less pronounced, with reduction seen only with respect to HF readmissions. Post-MI HF patients also benefit from early addition of MRAs. Guidelines strongly recommend usage of MRAs in these patient subsets to improve prognosis. Careful selection of patients and closer monitoring for hyperkalaemia may help maximise the adherence and, hence, benefits of MRAs in these high-risk patients. Newer nonsteroidal MRAs and aldosterone synthase inhibitors hold promise for improving safety and efficacy.