Heart failure (HF) is a major healthcare problem, thought to affect 64.3 million people worldwide based on a 2017 estimate; a number that has increased by 34% compared with 1990 and by 16% compared with 2007.1 It is projected that its prevalence will increase, owing primarily to ageing of the population, as the age-standardised prevalence of the disease has decreased.1,2 Specifically, HF prevalence is expected to increase by 46% between 2012 and 2030.3 HF also poses a significant societal burden in terms of resource use and healthcare expenditure. In 2012, the estimated total cost for HF was US$30.7 billion for the US alone and it is predicted that the cost will rise to US$69.8 billion by 2030, an increase of 127%.3,4
HF is a heterogeneous clinical syndrome, which has only recently been defined and classified universally.5 HF is currently divided into HF with reduced ejection fraction (EF; HFrEF), mildly reduced EF (HFmrEF) and preserved EF (HFpEF), based on EF ≤40%, 41–49% and ≥50%, respectively.5
Clinical research and treatment evidence in HFpEF is substantially limited compared to HFrEF, and this is reflected in the differences in numbers of class I indications for these two entities in the most recent guidelines.6,7
Diagnosis of HFpEF is challenging; gaps in our understanding of the disease’s pathophysiology, lack of universal diagnostic criteria and only modest sensitivity and specificity for criteria that are generally used, heterogeneity of HFpEF populations and presence of multiple noncardiac comorbidities contribute to this.8 Although natriuretic peptide and imaging data generally confirm the diagnosis, the Framingham criteria are met in only a quarter of patients.9 Therefore, HFpEF is an entity that often remains underdiagnosed.10,11 Even in the setting of specialised HF centres, data for HFpEF diagnosis are missing in three-quarters of the patients who have that label; when present, the diagnosis of HFpEF can actually be confirmed in only half the cases.12 This highlights how challenging it is to gather reliable data on HFpEF.
In this review, we aim to comprehensively describe the burden of HFpEF, providing contemporary data on epidemiology, clinical characteristics and comorbidity, cause-specific outcomes and costs, and make comparisons with its counterpart (HFrEF) when clinically relevant.
Epidemiology
Data on HFpEF epidemiology are sparse. Their reliability and generalisability are further hindered by the different manner in which HFpEF has been defined across studies, the different age cut-offs used in population selection that have major implications on prevalence and incidence calculations, the geographical and secular differences, and the fact that a HFpEF diagnosis is easily missed.13 The variability in the prevalence of HFpEF across different countries and left ventricular ejection fraction (LVEF) cut-offs used to define the entity are depicted in Figure 1.
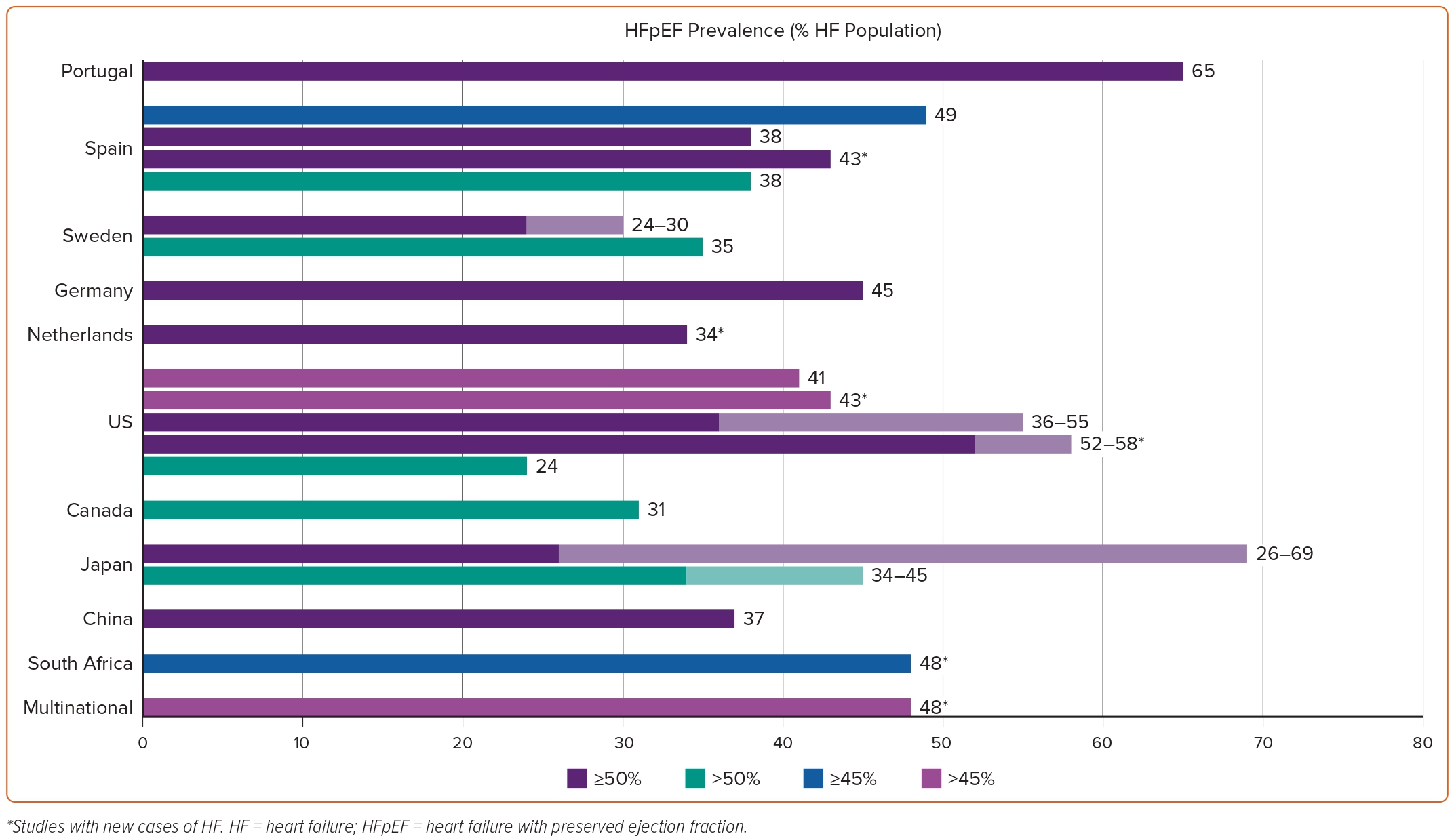
The relative contribution of different definitions of HFpEF in the variably reported epidemiology of the entity can be easily ascertained by a recent study among patients enrolled in the population-based Hamburg City Health Study.14 The application of the European Society of Cardiology 2021 versus the 2016 guidelines led to a decrease in the prevalence of HFpEF in the same patient population from 2.46% to 2.19%.
A summary of the main epidemiological studies across different geographical regions can be found in Supplementary Table 1.
North America
Most quality data on HF epidemiology are based on population studies performed in the US, where more than 6 million people over the age of 20 years currently have HF.3 Patients with HFpEF account for approximately 50% of incident HF hospitalisation cases, increasing over time from 33% in 2005 to 39% in 2010.3,15 Lifetime risk of HF in people aged >45 years ranges between 20% and 45%.3 Age and sex-adjusted incidence of HF has decreased over time, with a decrease being more prominent for HFrEF (45%) compared to HFpEF (28%).3
Four prospective, observational, community-based cohorts with adjudicated incident HF outcomes, three from the US and one from the Netherlands, were merged and analysed. Among 22,142 people aged >30 years, 1,666 presented with incident HF, of whom 795 (48%) were classified as HFpEF and 871 (52%) as HFrEF.16 Mean age at entry differed between the studies (49–73 years), as did the cumulative 12-year incidence of HF (4.2–13.7%).17 Frequencies of HF subtypes varied by cohort, with the proportion of incident HFpEF cases increasing in parallel with the mean age of the study population.17 These studies are outdated, having enrolled patients between 1979 and 2002 with follow-ups of up to 15 years. In this analysis, HFrEF and HFpEF were defined as EF ≤45% and >45%, respectively. Extrapolation of results to the contemporary era, which recognises HFmrEF as an important entity, is challenging.18–20
A recent study used electronic health records linked to claims data of individuals aged ≥65 years without known HF to study the incidence of HF in the community. The study followed 138,388 people for a mean of 3.4 ± 1.7 years and found an HF incidence rate of 20.9 (20.5–21.3) per 1,000 person-years; the respective rates for HF with preserved, reduced and uncertain EF were 6.1 (5.8–6.3), 2.0 (1.9–2.1), and 12.9 (12.6–13.2) per 1,000 person-years.21 Incidence rates of HFpEF increased considerably with age, from 3.1 (2.9–3.4) cases per person-year in the age group 65–69 years to 14.5 (13.6–15.4) cases per person-year in the age group >80 years.21 In this analysis, HFrEF and HFpEF were again defined as EF ≤45 and >45%, posing the same challenges in generalisation of results.
In terms of intertemporal changes in HF incidence, a study from several US community-based samples from 1990 to 2009 demonstrated that standardised HF incidence remained stable between the decades of 1990–1999 and 2000–2009 (from 19.7 [18.4–21.0]/1,000 people to 18.9 [17.7–20.1]/1,000 people, respectively). However, the incidence of HFpEF (EF ≥50%) significantly increased during the same period (from 4.7 [4.2–5.2]/1,000 people to 6.8 [6.1–7.5]/1,000 people). The increase in HFpEF incidence over this period was noted in both sexes.22
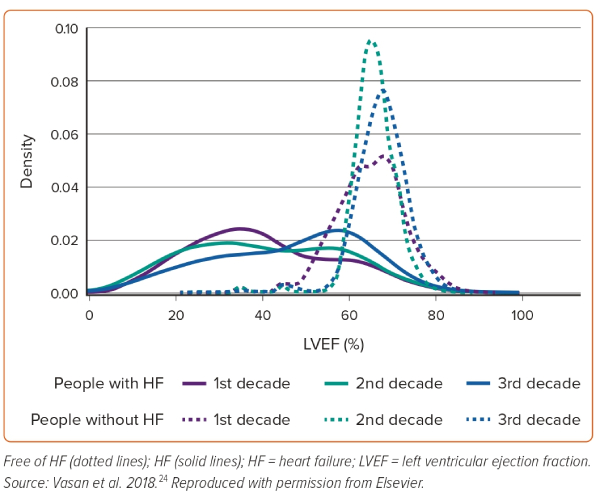
A recent analysis from Olmsted County, Minnesota, using the updated HF classification, reported that among 2,035 adults with incident HF between 2007 and 2015, 29.9% had HFrEF, 12.5% had HFmrEF and 57.6% had HFpEF.23 Similarly, an analysis from new-onset HF in the Framingham study across three decades (1985–2014) demonstrated increased frequency of HFpEF, decreased frequency of HFrEF and unchanged frequency of HFmrEF over time.24 The temporal changes over these three decades in the LVEFs among patients with and without HF are shown in Figure 2.24 This study underlines the increased diagnosis of HFpEF in the contemporary era. However, the degree to which this is attributable to a true increase in the incidence of the entity or increased awareness and better recognition of the disease by clinicians cannot be distinguished.
The average prevalence of HFpEF, expressed as a proportion of the total HF population, has varied considerably across studies, from 22.3% in a study from the early 1990s to 55% in a study from the Olmsted County population from the mid-2000s.25,26 However, it needs to be highlighted that an LVEF cut-off of 55% was used for defining HFpEF in the former study, which artificially drove the prevalence lower. An analysis from the Get with the Guidelines (GWTG)-HF cohort, including patients hospitalised with HF between 2005 and 2009, reported a HFpEF prevalence of 45.8%.27
Another analysis from the Olmsted County study provided insight into secular trends of HFpEF prevalence. Specifically, the prevalence of HFpEF cases as a proportion of the total HF hospitalisations steadily increased from 38% to 54% between 1997 and 2001. This was a result of an increase in the absolute number of HFpEF hospitalisations, while numbers of HFrEF admissions remained unchanged.28
In Canada, a study investigated the prevalence of HF subtypes among HF patients admitted for first HF hospitalisation across 103 hospitals in the province of Ontario between 1999 and 2001. Among the 2,802 patients included in the analysis, 31% had HFpEF (EF >50%) and 56% had HFrEF (<40%).29
Europe
In the landmark ESC HF Atlas, crude incidence and prevalence statistics were available for 12 (29%) and 13 (31%) participating countries, respectively. The median annual incidence of HF per 1,000 person-years was 3.20 and the median prevalence of HF per 1,000 people was 17.20 (IQR 14.30–21) cases.30 Unfortunately, no specific data on distribution of patients according to EF were available.
In a cross-sectional observational study among 5,434 subjects attending primary healthcare centres in 1998 in Portugal, the prevalence of HF with preserved systolic function in the population aged >25 years was 1.7%, compared with 1.3% for HF with systolic dysfunction; however, this estimation has little generalisability today as the echocardiographic criteria used in the study are now obsolete (i.e. LV shortening fraction <28% or severe LV dyskinesia/dilatation).31 In a more recent cross-sectional study of 126,636 subjects from the same country, the prevalence of HF was 2.13%, with HFrEF accounting for 16.3% and HFpEF for 65.4% of cases.32
In a study in Spain from 2004 to 2005, 6.8% of the study participants met criteria for congestive HF. The prevalence of HF with reduced and preserved systolic function, using an LVEF cut-off of 45%, was 3.5% and 3.3%, respectively.33 In another study performed in a single hospital between 2000 and 2007 in southern Spain, 43% of newly diagnosed HF cases had HFpEF, as defined by EF ≥50%.34 The overall incidence of HF increased over time, from 2.96 per 1,000 person-years in 2000 to 3.90 per 1,000 person-years in 2007. The prevalence of HF, both HFpEF and HFrEF (EF <50%), increased over time among both men and women.34 From 2000 to 2007, the prevalence of HF increased steadily from 0.9% to 2.1%. Data from the EPISERVE study also performed in Spain in 2005 raised the prevalence of HF in the community to 4.7%. Of these patients, 38% had an LVEF >50% and would most probably fulfil contemporary criteria for HFpEF. Consequently, the prevalence of HFpEF would be approximately 1.8% compared with 2.0% of HFrEF and 0.9% of HFmrEF.35 A more recent analysis from patients enrolled between 2013 and 2019 in a nationally representative, longitudinal database across Spain reported an HF incidence of 0.32 cases per 100 person-years and a prevalence of 2.34%, both of which increased every year.36 In 2019, 49.3% had HFrEF, and 38.1% had HFpEF. The considerable differences in the epidemiology of HF, even in studies performed in the same country and referring to the same period, further highlight how challenging it is to extrapolate and generalise these data.
In a study from Sweden of patients with at least two HF diagnoses in electronic medical files between 2010 and 2015 (n=8,702), information on HF EF category was only available for 3,167 (36.4%) patients; of these, 35.4% were classified as having HFpEF, defined as EF >50%.37 In another analysis from the Swedish HF Registry among 76,453 patients enrolled between 2005 and 2018, 53% had HFrEF (EF <40%), 23% had HFmrEF (40–49%) and 24% had HFpEF.38 Importantly, in another analysis from the registry, an increase in the proportion of HFpEF patients was reported from 20% in the period 2000–2004 to 30% in the period 2013–2016.39
Four studies performed in a primary care setting among high-risk community people aged 60 years or 65 years or older from the Netherlands were combined into a single data set. The studies only included older people with symptoms of exertional dyspnoea, polypharmacy or multimorbidity, chronic pulmonary obstructive disease, or type 2 diabetes.40 The prevalence of previously unrecognised left ventricular diastolic dysfunction or HFpEF was 64.2%, being higher in women than in men (72.2% versus 55.6%, respectively).40 Nonetheless, the decision of the investigators to pool asymptomatic left ventricular diastolic dysfunction and symptomatic HF does not permit identifying the proportion of people with HFpEF.40 In the PREVEND study, also performed in the Netherlands, the 12-year cumulative incidence of HF was 4.4%; of these people, 34% were diagnosed with HFpEF (EF ≥50%) and 66% with HFrEF (EF ≤40%).41
Asia
Most data from east Asia regarding HFpEF are derived from Japanese studies. Several multicentre studies have demonstrated that the prevalence of HFpEF among the overall HF population ranges from 26% to 69% in Japan.42,43
The weighted prevalence of HFpEF (EF ≥50%), HFmrEF (EF 40–49%) and HFrEF (EF ≤40%) in the Chinese population aged >35 years was shown to be 0.3, 0.3 and 0.7%, respectively, as assessed through a national survey performed between 2012 and 2015.44 Conversely, in a population-based study of 2,230 participants ≥35 years of age from rural areas of a specific Chinese province (Liaoning) performed between January 2012 and August 2013, HFpEF was diagnosed in 3.5% (1.8% in men and 4.9% in women) of the population.45 The prevalence of HFpEF increased with age in both sexes and was greater in women than in men for every age group. These discrepancies underline the importance of methodological approaches as well as regional disparities, even within the same country.
In a registry of 7,507 acute HF patients from Kerala, India (Cardiology Society of India-Kerala Acute Heart Failure Registry [CSI-KHFR]), the proportion of patients with HFrEF, HFmrEF and HFpEF was 67.5%, 17.6% and 14.9%, respectively, while the respective percentages in the National Heart Failure Registry (NHFR) that enrolled 10,851 acute HF patients across 53 tertiary care hospitals in 21 states in India between January 2019 and July 2020 were 65.2%, 22% and 12.7%, respectively.46,47
Africa
Data on HF prevalence in Africa are currently missing. In a hospital serving 1.1 million people in South Africa, 1,960 patients presented with HF in 2006; 43% had de novo HF and 48% of these had preserved EF, defined as LVEF ≥45%.48 A recent observational study from Tunisia registered HF patients, both in- and outpatients, treated by 250 cardiologists across multiple centres during October 2017. The study enrolled 2,040 patients, 80% of whom were outpatients. Importantly, patients with EF >50% consisted only 7.7% of the study population, 9.7% for inpatients and 7.1% for outpatients, likely underlining the inclination of cardiologists to diagnose and treat HFrEF over HFpEF patients, rather than true geographical variations in epidemiology.49
Characteristics of Patients with Heart Failure and Preserved Ejection Fraction
A recent meta-analysis identified a significant barrier to our knowledge of HFpEF patient clinical profiles. Reporting of comorbidities is much less common in HFpEF trials (only present in 27%) compared with trials in HFrEF (51%) and trials in HF regardless of the EF (48%).50 Collectively, the conditions that have demonstrated the largest increases in prevalence among HF patients over time include hypertension, AF and chronic kidney disease.50
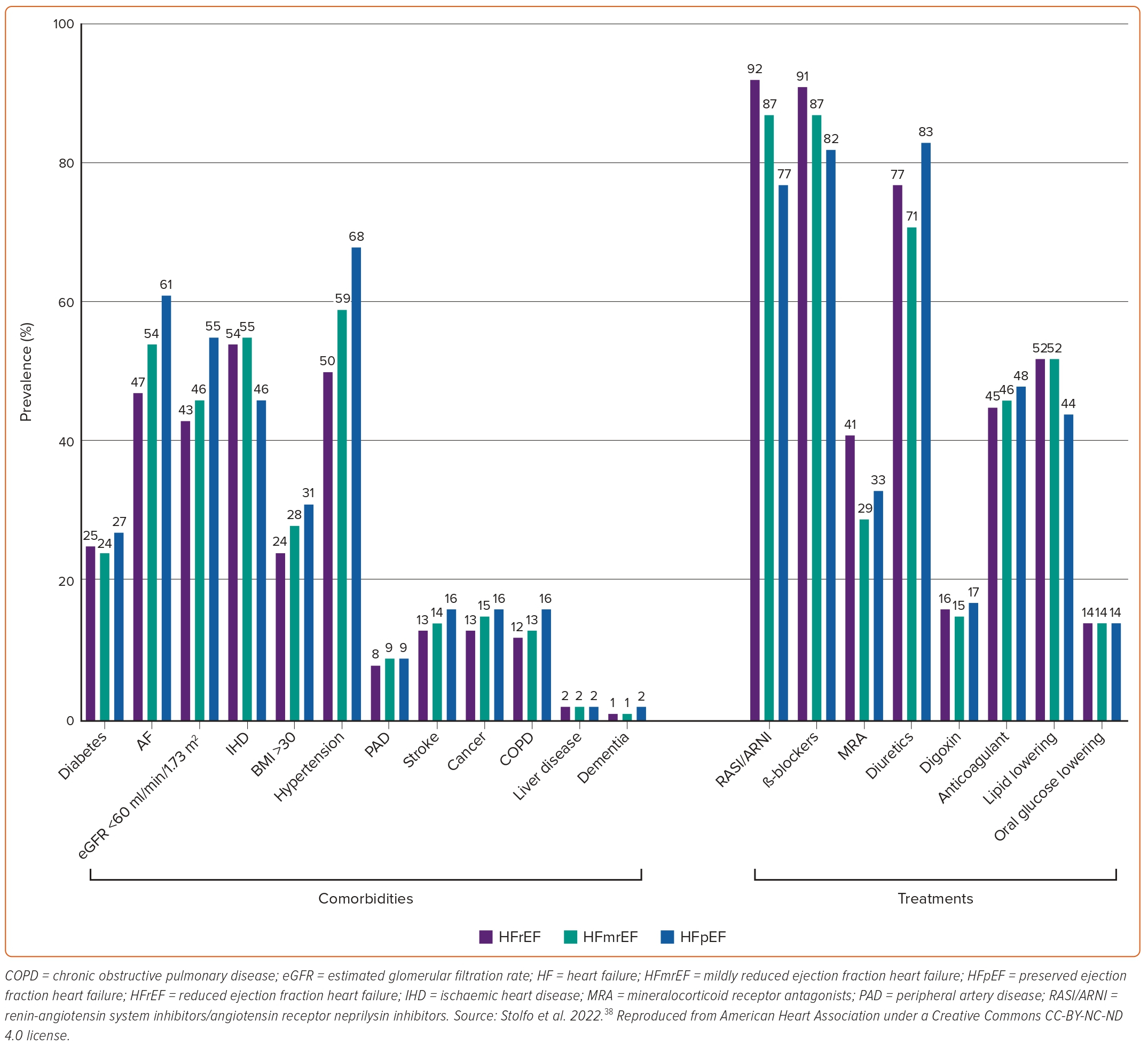
Common findings among several studies comparing HFpEF with HFrEF patients are that the former are older, more often women, and have an increased prevalence of hypertension, obesity, AF and anaemia.3,28,29,37,45,47,51,52 In terms of demographics, it has been reported that black people are less likely to have HFpEF compared with white people.52 Patients with HFpEF smoke less, but drink alcohol more often than patients with HFrEF.29,44,47 Regarding comorbidities, HFpEF patients have been reported to more often have chronic kidney disease, chronic pulmonary disease, and valvular heart disease compared with patients with HFrEF.3,28,29,37,44,47,51,52 In contrast, they less often have hyperlipidaemia, peripheral artery disease and cerebrovascular disease.29,37,45,52 Most studies have also demonstrated a reverse relationship between prevalence of coronary artery disease and HFpEF.28,29,37,45,52 However, a study from China showed the opposite, underlining the significance of regional variations.44 Most studies have demonstrated a neutral association between the presence of diabetes and HFpEF, though there have been studies indicating both a positive and negative association.29,45,47 Finally, patients with HFpEF have also been shown to have significantly lower levels of serum natriuretic peptides compared with their HFrEF counterparts.37,51 The prevalence of various comorbidities and of HF and non-HF treatments among patients with HF stratified by LVEF is shown in Figure 3.38
Outcomes
Mortality
A summary of the main mortality data across different geographical regions can be found in Supplementary Table 2.
North America
Nationwide statistics from the US suggest that 5-year mortality rates for patients with incident HF have remained stable at approximately 50% between 2000 and 2010.53 These data also demonstrated that cardiovascular (CV) death was more frequent among patients with HFrEF, while non-CV death was more frequent among HFpEF patients.3 In an older study from Olmsted County, the survival rate was higher among patients with HFpEF than among patients with HFrEF, although the difference was small (71% versus 68% at 1-year follow-up and 35% versus 32% at 5-year follow-up). Among patients with HFrEF, the likelihood of survival increased during the study period (1987–2001) but did not change significantly over time in HFpEF, possibly reflecting the uptake of disease-modifying treatments in the HFrEF population during this period and the lack of such treatments for HFpEF up to recently.28 Conversely, another analysis in the Framingham Heart Study and Cardiovascular Health Study populations did not demonstrate a secular difference in mortality for both HFrEF and HFpEF groups between the decades 1990–1999 and 2000–2009. However, the finding that HFpEF was associated with a lower risk of CV mortality but a higher risk of non-CV mortality compared with HFrEF was reproduced in this analysis.22 The incidence of all-cause mortality for patients with HFpEF in the ARIC study was 32.4 (25.3–41.5)/1,000 person-years.54 In another study from the US among patients hospitalised for HF, patients with HFrEF had a 39% and 25% increased adjusted risk of mortality at 30 days and at 1 year, respectively, compared with their HFpEF counterparts, a finding that was not confirmed in the OPTIMIZE-HF registry, where no difference in mortality risk between HF patients with reduced (<40%) and preserved EF (≥40%) was shown for after hospital discharge.55 However, despite the similar length of hospital stay, there was an increased risk for in-hospital mortality with EF <40% versus EF ≥40% (adjusted HR=1.28; 95% CI [1.13–1.46]; p=0.0002).56 In a recent analysis among almost 40,000 patients with mean age of 65 years, stratified into HFrEF (46%), HFmrEF (8%) and HFpEF (46%) and hospitalised with HF in the GWTG-HF cohort, a very high 5-year mortality rate of 75% was reported, which was independent of LVEF. Primary causes of death again varied across the EF spectrum, with patients with HFrEF being more likely to report cardiovascular death (65%) versus HFpEF (52%).27 In the same registry, among 110,621 patients admitted with HF from January 2005 to December 2010, 50% had HFrEF and 36% had HFpEF. In-hospital mortality for HFpEF decreased from 3.32% in 2005 to 2.35% in 2010 (adjusted OR=0.89/year; p=0.01) but was stable for patients with HFrEF (from 3.03% to 2.83%; adjusted OR=0.93/year; p=0.10).15
Among patients hospitalised for HF in Canada there was a trend towards lower 30-day (5.3% versus 7.1%, p=0.08) and 1-year mortality rates (22.2% versus 25.5%, p=0.07) in patients with HFpEF compared with HFrEF, an association that remained non-significant after adjustment for various confounders.29
Europe
In a study from Sweden among incident HF cases, HFrEF was surprisingly associated with a lower risk of 1-year all-cause mortality (HR=0.77; 95% CI [0.62–0.96]) when compared with the HFpEF subgroup. Nonetheless, the trend was the opposite for CV mortality, though the association did not reach statistical significance.37 In the nationwide Swedish Heart Failure Registry the adjusted risk for mortality in HFrEF was higher compared with HFpEF at 30 days (1.35; 95% CI [1.14–1.60]), 1 year (HR=1.26; 95% CI [1.17–1.35]) and 3 years (HR=1.20; 95% CI [1.14–1.26]).57 The PREVEND study from the Netherlands demonstrated that 5-year all-cause mortality was higher for subjects with incident HFrEF compared with incident HFpEF (p=0.038).41 A multicentre study from Italy demonstrated that adjusted mortality was lower in HFpEF versus HFrEF (HR=0.75; 95% CI [0.67–0.84]; p<0.001). HFrEF had the highest rates of cardiac death, whereas non-cardiac mortality was similar across EF categories. When adjudicating cause of death, non-cardiac causes accounted for 62% of all deaths in patients with HFpEF versus only 35% in patients with HFrEF.58 In the ECHOES study, which enrolled 6,162 patients with HF and/or left ventricular systolic dysfunction in the UK, 5- and 10-year survival was 53% and 27%, respectively. The 10-year survival was 76.1% in patients with EF ≥40% and only 30.8% in patients with EF <40%.59
In the EuroHeart Failure Survey I, performed in 115 hospitals from 24 ESC member countries in the early 2000s, 90-day mortality following an HF hospitalisation was 12% in HFrEF and 10% in HFpEF (HR=1.35; 95% CI [1.13–1.62]).60 A more recent analysis from the ESC-HF-LT registry, performed more than a decade later in both inpatients and outpatients, reported that 1-year mortality was again higher in HFrEF (8.8%) versus HFpEF (6.4%, p=0.0002). In line with previous observations, the proportion of CV deaths at 1 year was higher, though not significantly, in patients with HFrEF (53.5%) compared to patients with HFpEF (47.2%).61 In a more recent analysis from the same registry focusing only on patients admitted for acute HF the authors reported higher inpatient mortality for patients with HFrEF (3.4%) compared to patients with HFpEF (2.2%, p=0.01).62 During 1-year follow-up, patients with HFrEF had higher rates of all-cause (22 [20–24] versus 17 [15–20]) and CV death (12 [10–13] versus 8.4 [6.9–10]) but lower rates of non-CV death (2.4 [1.8–3.1] versus 4.5 [3.5–5.9]) compared with HFpEF.62 In another study enrolling ambulatory HF patients from north-western Europe (Norway, Germany and the UK), long-term survival was independent of LVEF category after adjusting for a wide range of covariates. Interestingly, mortality rates among HF patients improved between 1995–2005 and 2006–2015 (HR=0.81; 95% CI [0.72–0.91]; p<0.001).63
Asia
Contemporary data on outcomes in HF across Asia are limited. In-hospital mortality of HFpEF patients was 4.6% in Thailand and 2.2% in Singapore.42 The CHART studies showed that all-cause mortality of Japanese patients with HFpEF at 1, 2 and 3 years was 7%, 16% and 22%, respectively.64 HFpEF patients had in-hospital mortality of 6.5%, which was not different from patients with reduced EF (<40%) after multivariable adjustment. There was no significant difference in survival analysis for all-cause mortality or cardiac mortality between patients with HFpEF and HFrEF. Similar results were reported from the JCARE-CARD registry enrolling 2,675 patients from 164 hospitals.65 In a prospective cohort study of 4,056 patients hospitalised for acute decompensated heart failure in Japan from October 2014 to March 2016, over a median follow-up period of 470 (IQR 357–649) days, all-cause mortality was 21.5% for HFrEF and 24.0% HFpEF (p=0.26), CV mortality was 14.7% in HFrEF and 13.7% in HFpEF (p=0.71), and sudden cardiac mortality was 3.2% in HFrEF and 2.5% in HFpEF (p=0.23).66 In the prospective multicentre China-HF Registry enrolling hospitalised patients with HF, crude in-hospital mortality was 4.1% and significantly higher in patients with HFrEF versus HFpEF (4.0% versus 2.4%, respectively) when a cut-off LVEF of 45% was used.67 In the Asian-HF registry, crude 1-year all-cause mortality was 9.6%. Asian patients with HFrEF had higher 1-year mortality than those with HFpEF (10.6% versus 5.4%). CV causes of death were slightly higher in HFrEF (54.0%) versus HFpEF (53.3%), whereas non-CV death was higher in HFpEF (23.3%) versus HFrEF (12.0%) at 1 year. One-year all-cause mortality was highest in southeast Asian patients (13.0%), followed by south Asian patients (7.5%) and north-east Asian patients (7.4%).68
In the CSI-KHFR from India, the in-hospital and 90-day mortality rates were higher in patients with HFrEF (7.7% and 12.3%) compared with patients with HFmrEF (5.2% and 9.9%) and HFpEF (5.6% and 10.6%), respectively.46 In the NHFR from the same country the rates of in-hospital mortality were 7.5%, 5.1% and 5.5%, and the rates of 90-day mortality 15.7%, 11.0% and 12.5% for HFrEF, HFmrEF and HFpEF, respectively.47
Australia
A snapshot study from 16 hospitals enrolling patients in the Victorian Cardiac Outcomes Registry reported similar inpatient and 30-day mortality for patients with HFrEF (4.2% and 8.0%, respectively) and HFpEF (4.8% and 8.3%, respectively).69
International Data
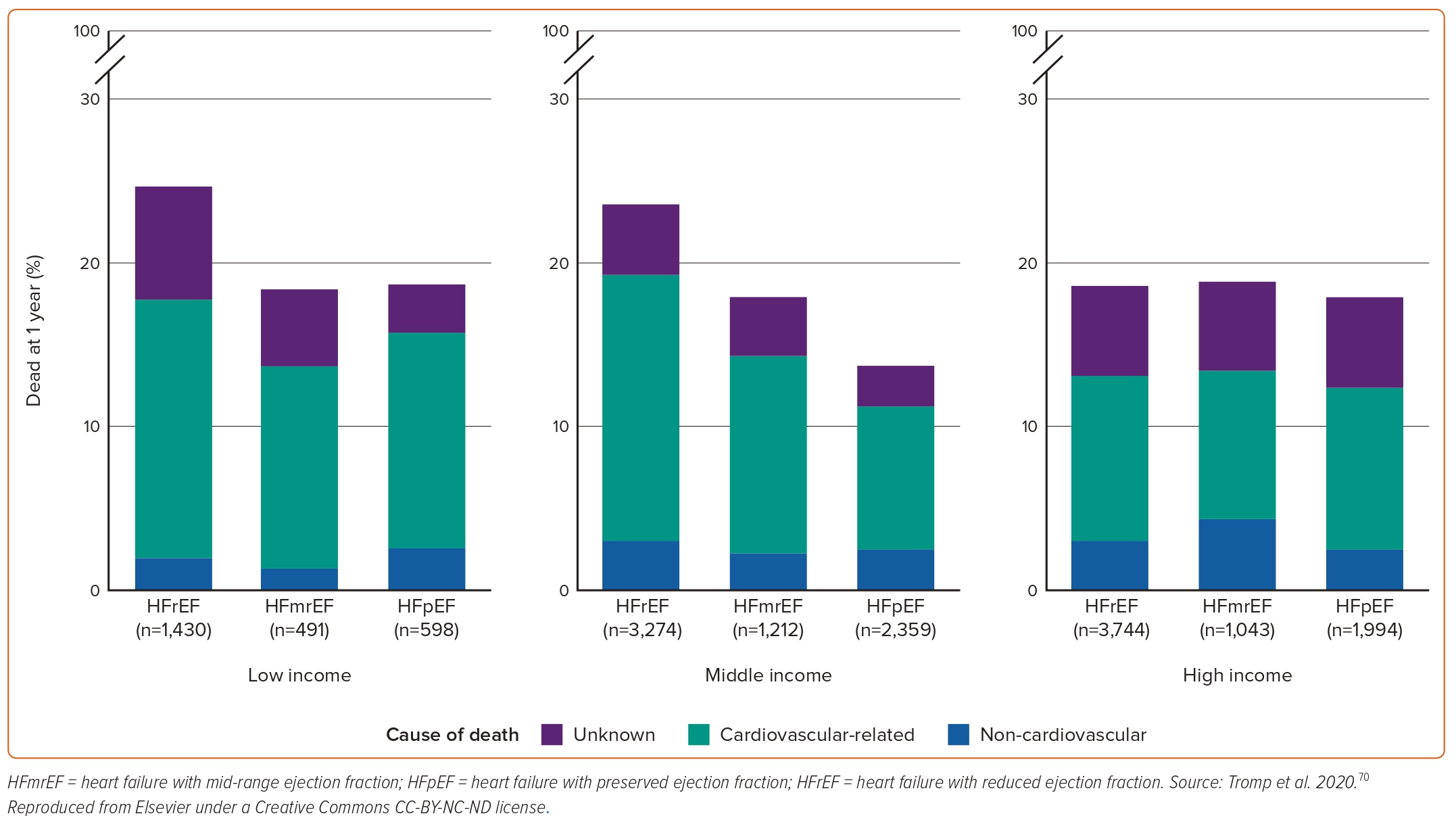
In REPORT-HF, which enrolled 18,102 acute HF patients from 358 centres in 44 countries, HFpEF patients had lower rates of 1-year mortality compared to patients with HFrEF across all country income levels (Figure 4).70 HFpEF in this study was independently associated with better 1-year survival compared with HFrEF (0.67; 95% CI [0.61–0.74]; p<0.001).70 The Global Group in Chronic Heart Failure (MAGGIC) study, pooling data from 31 observational studies and clinical trials, showed that patients with HFpEF were at a 32% lower adjusted risk of death compared to their HFrEF counterparts (22% lower adjusted risk after exclusion of clinical trials).71 In a systematic review analysing data from 1.5 million patients with chronic or stable HF in any ambulatory setting from 60 studies across high-income countries over the past 70 years, 5-year survival with HFrEF was lower compared with mixed EF, but there was no difference in survival for HFpEF compared with HFrEF at 1 and 5 years.72
The INTER-CHF study enrolled 5,823 patients between 2012 and 2014 and assessed 1-year mortality in patients with HF in Africa, China, India, the Middle East, southeast Asia and South America. Patients with HFrEF accounted for 50% of the population, with wide variations between 39% in southeast Asia and 73% in the Middle East. Overall mortality rate was 16.5% but ranged significantly from 7% in China up to 34% in Africa. HFrEF was not associated with increased risk of mortality after adjustment for covariates.73
Even within the heterogeneous HFpEF population, outcomes are not uniform. Machine learning techniques have recognised three different phenocopies within this population, with the patients in the phenogroup with significantly higher BMI, more severe HF symptoms, and higher burden of diabetes, dyslipidaemia, and atherosclerotic CV disease having worse outcomes compared with the rest of HFpEF patients.74
In the most recent randomised controlled trials investigating the effect of various drugs (spironolactone, empagliflozin and dapagliflozin) versus placebo on outcomes in patients with HFpEF, the incidence of death in the control arms ranged from 4.6 to 7.6 per 100 patient-years.75–77 However, the generalisation of these results is challenging because these trials included patients with different LVEFs cut-offs encompassing at least in part patients with HFmrEF, used inclusion criteria for enrichment reasons (e.g. age >40 years, elevated natriuretic peptides, presence of structural heart disease, etc.) or exclusion criteria for safety reasons (estimated glomerular filtration rate (eGFR) <30 ml/min per 1.73 m2 of body-surface area), and only enroled patients in highly selected sites and countries, which is typically the case in randomised controlled trials.
Hospitalisations
Heart failure was the second most common primary discharge diagnosis in the US for 2018, representing 3.2% of all hospital admissions.78 It may seem that 3.2% of hospitalisations is low, but this may reflect inclusion of elective hospitalisations and procedures in the total hospitalisations denominator. HF is the single most common cause of hospitalisation among people aged over 65 years.79 Approximately 30–40% of HF patients have a history of prior hospitalisation for HF, and up to 50% are (re-)admitted within 1 year of initial diagnosis of HF.55,80–82 In contrast to mortality data that have been contradictory, the vast majority of evidence across variable settings indicate that the risk of hospitalisation in HF is independent of EF and that patients with HFpEF are at a similarly high risk of (re-)hospitalisation as their HFrEF counterparts.27,29,55,56,60,83
Similarly, a study from the Olmsted County cohort demonstrated that hospitalisations in patients with HF had an incidence rate of 1.34 per person-years and were mostly due to non-CV causes (63%). Total hospitalisation rates were similar regardless of EF, with some evidence of a higher rate of CV hospitalisations among HFrEF offset by a higher rate of non-CV hospitalisations among HFpEF patients.53 A similar finding was recently reported by two studies performed in Sweden.37,84 All-cause readmissions were higher in patients with HFpEF, whereas HF readmissions were higher in patients with HFrEF. The incidence of several relevant outcomes in a population of patients with HF stratified by EF category is shown in Figure 5.38In terms of secular trends, the latter Swedish study showed that between 2004 and 2011, HF readmission rates within 6 months (from 22.3% to 17.3%, p=0.003) and 1 year (from 27.7% to 23.4%, p=0.019) declined significantly for HFpEF patients, and the risk reduction of 1-year HF readmission remained significant after adjusting for age and sex (adjusted HR=0.93; 95% CI [0.87–0.99]; p=0.022). However, no significant changes were observed in all-cause or CV readmission rates in HFpEF, and no significant changes in cause-specific readmissions were observed in HFrEF overall.
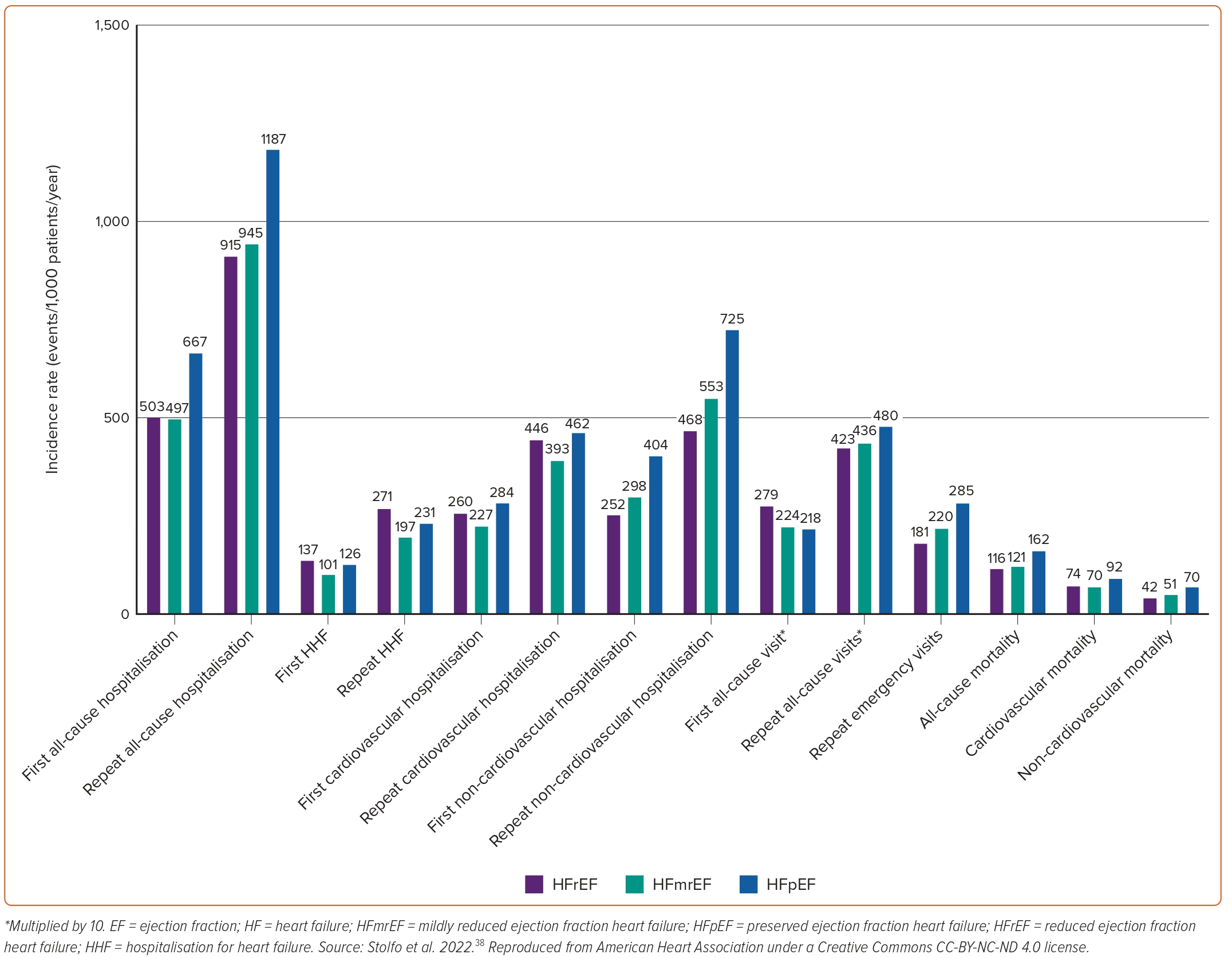
However, a different pattern was observed regarding hospitalisations at 1 year in patients enrolled in the ESC-HF-LT Registry, in which HFpEF patients presented with significantly lower rates of both all-cause (23.5% versus 31.9%) and HF hospitalisations (9.7% versus 14.6%) compared with their HFrEF counterparts.61 The same trends were noted in the registry when only the acute HF subpopulation was analysed.62 Similarly, in the CHARM programme HFrEF was associated with an increased risk of HF hospitalisations when compared with HFpEF (1.42 [1.23–1.64]; p<0.001), after adjustment for multiple covariates.85 These differentiations may possibly reflect the highly selected populations followed in specialised HF centres and clinical trials, respectively.
Costs
The global economic burden of HF is estimated at US$108 billion per annum, with the most significant economic costs of HF deriving from hospitalisations.86–88
The gaps in the systematic diagnosis of HFpEF in the community and the paucity of reliable data on its epidemiology, mentioned above, explain, to a large extent, why data on resource use and costs related specifically to the management of HFpEF patients are extremely limited. A relevant systematic review accrued 16 international studies published between 2004 and 2016 on this topic.86 Although there was significant variation in annual costs of HFpEF among the included studies, ranging from as low as US$868 in South Korea to as high as US$25,532 in Germany, the authors estimated that the overall lifetime healthcare costs for HFpEF were US$126,819 per patient.86
A more recent systematic review focused on studies investigating only in-hospital costs of HFpEF patients identified nine such studies published between 2001 and 2020, six of which were performed in the US.89 Based on calculations for the financial year 2019, the mean cost of index HF hospitalisation ranged between US$8,340 and US$11,366 per admission but rose as high as US$31,493 per admission in patients with comorbidities. Only two studies among the ones included in the meta-analysis reported data on 1-year costs; these were US$26,343 and US$27,174, respectively.89 Another systematic review, which included 23 studies between 2014 and 2019 that assessed cost per HF hospitalisation in the US, reported a mean cost of US$10,737–17,830 per HF hospitalisation.90 For patients with HFrEF or HFpEF, mean cost range was US$11,600–17,779 and US$7,860–10,551, respectively.90
Two recently published analyses provide further insight on the topic as they were not included in the systematic reviews above. The first, which was based on the ALDO-DHF trial that enrolled HFpEF outpatients in Germany and Austria between 2007 and 2011, showed that the mean and median annual costs per patient were €1,118 ± 2,475 and €332, respectively.91 This analysis also confirmed that main driver of cost in these patients was hospitalisation and that the presence of comorbidities, such as anaemia, coronary artery disease and AF, were independently associated with increased healthcare costs.91 The cost of treatment in this cohort was lower than that reported for HFrEF patients. However, the population of HFpEF included in this study was unique as it consisted of relatively young and oligosymptomatic patients, resulting in a low rate of HF hospitalisations (only 14.7% of patients during follow-up) and a low consequent cost. Therefore, its results are not readily generalisable to the unselected HFpEF population. In the other study from the US, 109,721 HF patients (22% HFrEF, 31% HFpEF and 47% unclassified EF; median 18 months follow-up) were identified using claims/electronic health records from July 2012 through June 2018.92 Mean number of all-cause outpatient visits per patient-month was 3.3 for HFrEF and 3.6 for HFpEF patients, while 11% of patients experienced a HF hospitalisation with a significant higher rate among HFrEF (23%) versus HFpEF patients (16%). Average total direct healthcare costs per patient-month were US$9,290 and was significantly higher among HFrEF (US$11,053) compared with HFpEF patients (US$7,482).92
In a study from Spain including 21,297 patients, the annual healthcare resource cost of HF patients was €3,193.2 ± €4,457.7. The annual cost was significantly higher for patients with HFrEF (€4,358.7 ± €5,522.8) compared with patients with HFpEF (€2,086.1 ± €2,737.8).93 In another study from Turkey the total direct annual cost for patients with HF was US$887, while the respective sums for patients with HFrEF and HFpEF were US$1,147 and US$649, respectively.94
Finally, a study from a single centre in Japan reported that median hospitalisation costs (in 2017 US dollars) were similar in the HFrEF and HFpEF groups (US$7,128 versus US$6,580, respectively, p=0.189).95 However, when focusing only on patients aged ≥75 years, median hospitalisation costs were significantly higher in the HFrEF (US$7,240) relative to the HFpEF group (US$6,471; p=0.014).95
Unfortunately, the data on costs are limited and specific to the countries on which these analyses were based. It is noteworthy that in many underdeveloped and developing countries, especially ones with a strong manufacturing base, drugs are supplied at a fraction of the cost of those in developed countries. Similarly, cost of hospitalisation in these countries is also substantially lower, as is the income per capita, making extrapolation of cost-effectiveness analyses from the US or Europe extremely challenging. Especially for the US, healthcare-related expenditures are so much higher than in other countries, that it does not seem reasonable to use US data to inform policies in other countries.96
Pharmacotherapy
In contrast to HFrEF in which quadruple therapy with renin-angiotensin system inhibitors (RASis), β-blockers, mineralocorticoid receptor antagonists (MRAs) and sodium–glucose cotransporter 2 inhibitors (SGLT2is) is well established and strongly recommended in the latest scientific society guidelines, the drug options for patients with HFpEF are sparse.97,98 Until recently, all major randomised controlled trials in patients with HFpEF failed to achieve their primary endpoints.97 However, signals of efficacy in reducing hospitalisations were noted in secondary analyses of trials with candesartan, spironolactone and sacubitril/valsartan, securing them a weak recommendation for patients with HFpEF in the latest US but not European guidelines.75,85,97–102 The seminal EMPEROR-Preserved trial randomised nearly 6,000 symptomatic patients with HF and EF >40% to receive empagliflozin (10 mg once daily) or placebo, in addition to usual therapy.76 Empagliflozin led to a significant 21% reduction in the incidence of the primary outcome, which was the composite of CV death or hospitalisation for HF. These results, which were mainly driven by a reduction in the incidence of HF hospitalisations, were consistent across several pre-defined subgroups, including the subgroup of patients with EF <50% and 50–60%. These findings were reproduced in the DELIVER trial, which randomised 6,263 patients with HFpEF or HFmrEF and increased natriuretic peptides to receive dapagliflozin 10 mg once daily or placebo. Dapagliflozin significantly reduced the risk of CV death and/or worsening HF by 18% by significantly reducing the incidence of worsening HF (HR=0.79; 95% CI [0.69–0.91]), but not CV mortality (HR=0.88; 95% CI [0.74–1.05]). The findings were again consistent across EF subgroups (<50%, 50–59%, ≥60%).77 Thus, SGLT2is are the first drugs to uniformly improve the outcomes of patients with HF across the EF spectrum and represent the cornerstone of pharmacotherapy in patients with HFpEF.
Conclusion
HFpEF is a major healthcare problem. Given the ageing of the population, its significance in terms of prevalence and usage of resources and healthcare costs is due to surpass that of its counterparts, HFrEF and HFmrEF. Better understanding of the disease’s pathophysiology, unification of diagnostic criteria, gathering of epidemiological data on a regional level and meaningful phenotyping of the heterogeneous HFpEF population are warranted to implement measures that can improve patient prognosis and reduce the societal burden of the disease. To this end, research in HFpEF should be prioritised. The recent positive trials with SGLT2is in HFpEF have paved the way. 










