Sacubitril/valsartan (S/V) therapy in patients with heart failure (HF) with reduced ejection fraction (HFrEF) has been shown to be superior to enalapril in reducing the risk of death and hospitalisation for HF.1 Cardiopulmonary exercise testing (CPET) is a powerful predictor of mortality in HF patients. Recent studies have demonstrated a significant symptomatic and functional improvement following the initiation of S/V therapy.1–6 Moreover, S/V treatment was reported to attenuate cardiac remodelling and dysfunction, inhibit fibrosis and reduce hypertrophy in a rat model of HF after MI.7 S/V treatment also prevented maladaptive cardiac fibrosis and dysfunction in a mouse model of left ventricular (LV) pressure overload.8 However, the molecular mechanism of this pleiotropic effect is not fully understood. The present study aims to evaluate the effects of S/V on functional capacity in HFrEF patients and whether myocardial fibrosis, assessed by cardiac magnetic resonance (CMR), influences cardiopulmonary functional capacity responses in HFrEF patients after S/V therapy.
Methods
This was an observational, prospective study conducted in patients with HFrEF. All patients signed informed consent at enrolment. The inclusion criteria were as follows: New York Heart Association functional class (NYHA) class II–III, ejection fraction <35%, treatment with an optimal dose of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor antagonists (ARBs) in the previous 6 months, systolic blood pressure >100 mmHg, serum potassium level <5.4 mEq/l, estimated glomerular filtration rate (eGFR)>30 ml/min/1.73m2, absence of severe hepatic insufficiency (Child-Pugh class C) and a negative history of angioedema. The exclusion criteria were as follows: hospitalisation for HF in the 90 days prior to the outpatient evaluation, myocardial revascularisation in the 180 days before the outpatient evaluation, concomitant initiation of cardiac resynchronisation therapy (CRT) and/or percutaneous mitral valve treatment or within the previous 6 months, congenital heart disease and inability to perform CPET.
The following demographic, anthropometric and clinical data were collected in an anonymised database for every patient: date of birth; race; sex; weight; height; BMI; body surface area (BSA); comorbidities (e.g. arterial hypertension, dyslipidaemia, chronic obstructive pulmonary disease, coronary artery disease, chronic renal failure); and previous pacemaker, ICD or CRT device implantation; Echocardiographic and CMR data (when available); LV end-diastolic diameter (measured in parasternal long axis projection) and the ejection fraction measured by the Simpson biplane method were evaluated on the echocardiogram. Diastolic function was assessed by pulsed and tissue Doppler. Left atrium volume and area were evaluated in four apical chambers projection. Pulmonary systolic arterial pressure was obtained from the sum of the pressure gradient between the right atrium and the right ventricle (calculated from the maximum speed peak of the tricuspid regurgitation) and pressure in the right atrium (estimated based on the size and collapsibility of the inferior vena cava). Tricuspid annular plane systolic excursion was interpreted according to current guidelines.9
CMR was performed in a subset of study patients who had not yet undergone ICD/CRT implantation using a 1.5-T scanner (TwinSpeed EXCITE, GE Healthcare). Data relating to LV mass, maximum wall thickness, biventricular and biatrial volumes and ejection fraction were collected and evidence of delayed enhancement (DE) was evaluated after administration of paramagnetic contrast medium intravenously (0.2 ml/kg of gadolinium). The presence and distribution of late gadolinium enhancement (LGE) were determined by signal-intensity-based quantitative analysis. The extent of LGE, defined by a signal intensity >5 SDs above the mean of the reference region of interest (ROI), was quantified on the short-axis contrast images. The extent of LGE was expressed as a weight of tissue and as a percentage of LV mass (indexed LGE extent).
S/V was prescribed according to current European guidelines.10 Drug titration was performed every 4 weeks (if tolerated by the patient). Changes in the dosage of diuretics during follow-up were made only when clinically necessary.
A baseline CPET was performed prior to initiation of S/V therapy and was repeated at 3, 6, 12 and 24 months. All CPETs were conducted on a cycle ergometer with a mean pedalling frequency of 60 rpm. A ramp protocol was performed in all patients, with a base workload of 10 Watts for 2 minutes and an increment of 10 W every 60 seconds. CPET was performed using the VMax2900 device (SensorMedics). The 12-lead ECG and oxygen saturation (measured by pulse oximeter) were constantly monitored during the test. Patients were encouraged to continue exercising until the onset of muscle exhaustion and/or dyspnoea. The anaerobic threshold was measured by V-slope analysis, based on carbon dioxide production (VCO2) and oxygen consumption (VO2), and confirmed by ventilatory equivalents and end-expiratory pressures of CO2 and O2. The percentage at which VO2 increased per Watt of work (ΔVO2/Δ work) was calculated for the progressive increase in exercise, starting 1 minute after the percentage of work began to increase. Finally, the relationship between minute ventilation and carbon dioxide production slope (VE/VCO2 slope) was calculated from 1 minute before the beginning of the workload to the end of the isocapnic breathing period.
For the calculation of the ratio between dead space volume and tidal volume (RV/VT), Jones’s prediction equation was used.11 Values for VO2, ventilation, tidal volume and RV/VT ratio at peak exercise (calculated through a non-invasive assessment of blood pressure of carbon dioxide) were extrapolated as an average greater than the 30 seconds in which the examination was carried out.
Statistical Analysis
Statistical analysis was performed using SAS JMP pro 9.0 software version. Continuous variables are described as mean ± SD and as a median and interquartile interval in the case of non-normal distribution. Categorical variables were expressed in numbers (percentages). The baseline (at time zero) and on-going follow-up parameters of the cardiopulmonary test were compared using the Mann-Whitney U-test for continuous variables and the Fisher’s exact test for categorical variables, respectively. McNemar’s paired t-test was used for analysing changes from baseline. Univariate and multivariate logistic regression analyses were performed to identify the predictive role of clinical variables for S/V therapy undergoing CMR. All cut-offs for continuous variables were obtained through the analysis of the receiver operating characteristic (ROC) curves. A p<0.05 was considered statistically significant.
Results
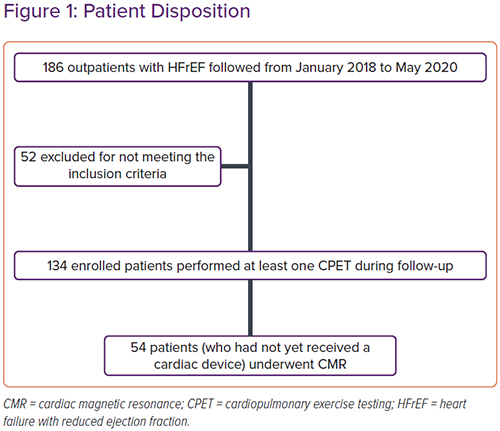
From 1 January 2018 to 31 May 2020, 186 outpatients with HFrEF were evaluated in the centres involved in the study. Fifty-two patients were excluded: 16 treated with ACEI for <6 months, 10 with eGFR <30 ml/min/1.73 m2, five with severe hepatic insufficiency, 11 with a history of recent hospitalisation for acute HF, eight unable to perform CPET and two who underwent MitraClip surgery in the previous 6 months (Figure 1). Data from 134 patients were analysed. All patients underwent at least one follow-up visit with CPET at 3, 6, 12 or 24 months.
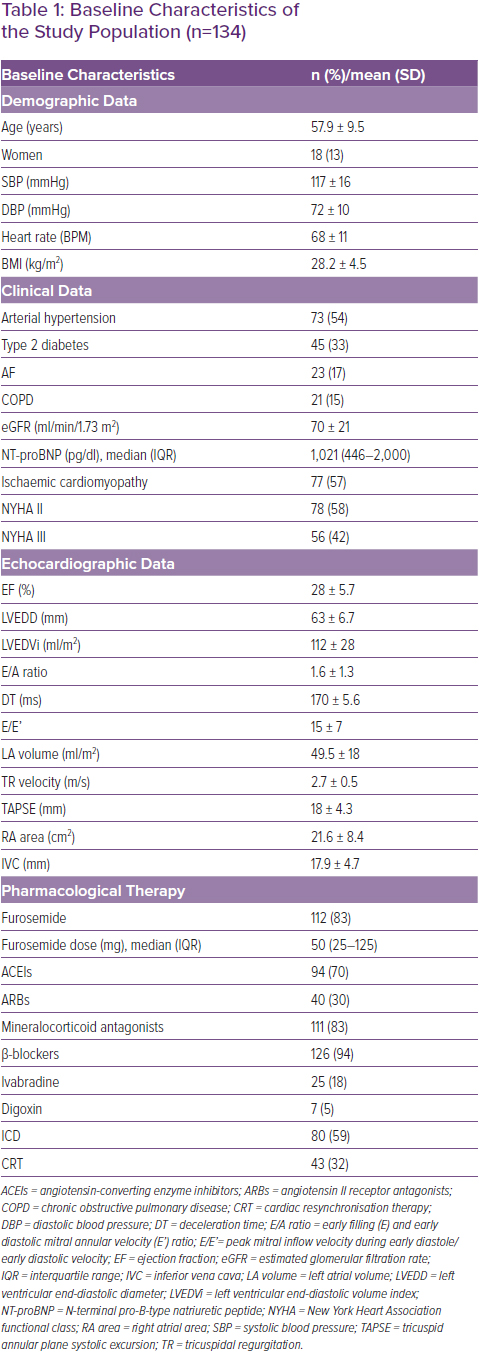
Table 1 shows the demographic, clinical, echocardiographic and pharmacological data of the population under examination. The average age of the studied sample was 57.9 ± 9.5 years and 13% of the subjects were female. Seventy-seven patients (57%) had chronic ischaemic heart disease. At the time of enrolment, 78 patients (58%) were in NYHA class II and 56 (42%) in NYHA class III. The mean LV ejection fraction (LVEF) was 28 ± 5.7% (Table 1). The starting dose of S/V was 24/26 mg twice daily in 72% of patients. After titration of S/V, during the follow-up (mean follow-up 13.3 ± 6.6 months), 29% of patients received the 24/26 mg dose, 35% the 49/51 mg dose and 36% the 97/103 mg dose.
During follow-up there was improvement in LVEF(p=0.0003), a significant reduction in systolic blood pressure (from 117 ± 16 mmHg to 103.1 ± 13 mmHg; p<0.0001), a reduction in E/A ratio (p=0.007) and in the size of the inferior vena cava (p=0.009).
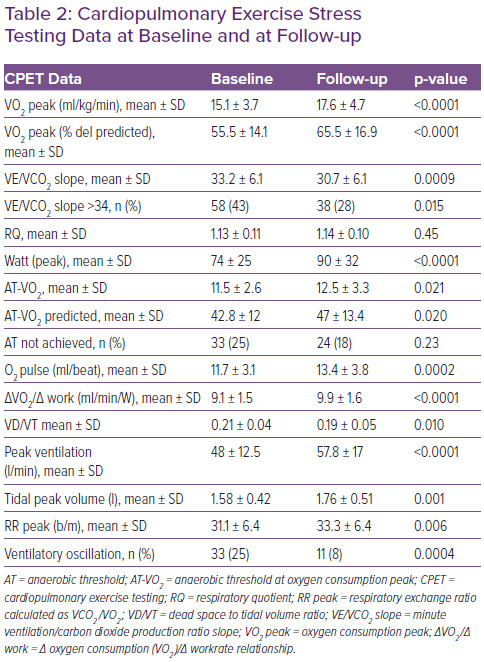
Table 2 shows the CPET data at baseline and at follow-up. At baseline, most patients were classified into the second ventilatory class and Weber class C. During follow-up there was an increase of 16% in VO2 peak (Δ = +2.5 ml/kg/min; p<0.0001) and of 13% in O2 pulse (Δ = +1.7 ml/beat; p=0.0002), as well as an improvement in ventilatory response associated with a 7% reduction in the VE/VCO2 slope (Δ =-2.5; p=0.0009). VO2 at the anaerobic threshold (AT-VO2) changed from 11.5+2.6 to 12.5+3.3 ml/kg/min (p=0.021); in addition, an increase of 8% in the ΔVO2/Δ work ratio (Δ = +0.8 ml/beat; p<0.0001) and of 18% in exercise tolerance (Δ = +16 Watt; p<0.0001) were registered.
During follow-up visits, significant reduction in mean N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels (from 1021 to 570 pg/ml; p=0.007) was observed.
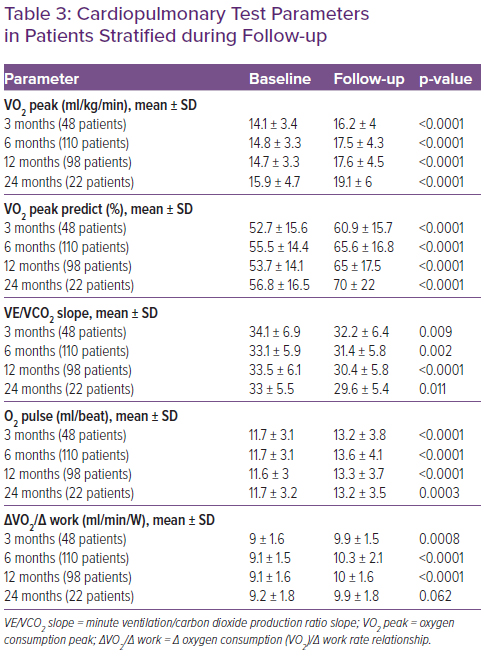
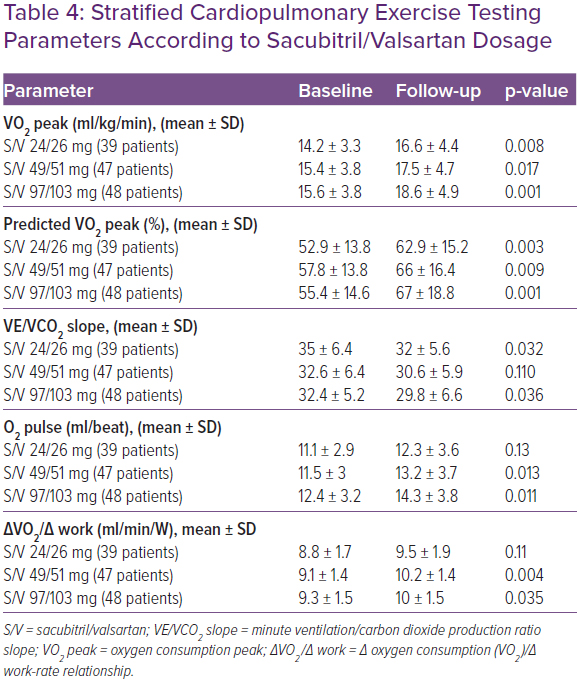
Table 3 shows the CPET data in baseline conditions and during the follow-up at 3, 6, 12 and 24 months. CPET parameters were assessed during follow-up by stratifying patients according to S/V dose. An improvement in VO2 peak and in predicted VO2 max, plus a reduction in VE/VCO2 was observed in all patient categories. In patients in whom S/V was administered at dosages of 97/103 mg twice daily and 49/51 mg twice daily, the observed increase in O2 pulse and in VO2/work was greater than the group of patients taking the lowest dose (Table 4).
Patients were observed for a mean follow-up of 13.3 ± 6.6 months. Fifty-six events were observed during the follow-up: 43 hospitalisations for HF (32%), two LV assist device implants (1.4%), two heart transplants (1.4%) and nine deaths from cardiovascular disease (6.7%).
In the multivariate logistic regression analysis, the main predictors of events during follow-up were VE/VCO2 slope >34 (OR 3.98; 95% CI [1.59–10.54]; p=0.0028), a ventilatory oscillatory pattern (OR 4.65; 95% CI [1.55–16.13]; p=0.0052), and haemoglobin (OR 0.35; 95% CI [0.21–0.55]; p<0.0001).
The CMR study was conducted in 54 patients who had not yet undergone ICD or CRT implantation at the time of enrolment. The demographic, clinical, anamnestic and instrumental features of the studied subgroups were comparable to those of the general population. Analysing the magnetic resonance data, the ejection fraction was comparable to that calculated by the echocardiogram (28 ± 6% versus 28 ± 5.7%); the indexed mass of the LV had an average value of 83 ± 22 g; the mass of DE in g was equal to 8 (0–25.7); the % of DE was 10.9 ± 14.4% and the DE index was 3.9 (0–12.8). Supplementary Material Table 1 shows the CMR data.
ROC curve analysis showed that a DE cut-off of >4.6% is the best predictor of events during follow-up (AUC 0.65; sensitivity 71%; specificity 63%).
In patients with a DE>4.6%, a lower response to S/V therapy was observed, showing lower improvement in Δ VO2 peak (+2.1 l/min versus +4.7 l/min), O2 pulse (+1.4 ml/beat versus+4.2 ml/beat), ejection fraction (+4.1% versus +10%), and NT-pro BNP (760 versus 810 pg/ml), whereas no significant differences were observed in ΔVO2/Δ work and VE/VCO2 slope (Supplementary Material Table 2).
In the cohort of patients undergoing CMR, 20 events (four deaths and 16 hospitalisations) were recorded during a mean follow-up of 14 ± 7 months. Both univariate and multivariate logistic regression analyses were also performed in the subgroup of patients undergoing CMR. In the multivariate analysis, the main predictors of cardiovascular events during follow-up were VE/VCO2slope (OR 1.42; 95% CI [1.19–1.82]; p<0.0001) and the % of DE (OR 1.13; 95% CI [1.02–1.36]; p=0.0087).
Discussion
The present study showed that S/V improves cardiopulmonary functional capacity by 16% despite the presence of myocardial fibrosis at CMR in a substantial proportion of patients. The CPET is a useful diagnostic and prognostic tool in patients with HF with both reduced and preserved ejection fraction.12–15 In the PARADIGM-HF trial, S/V was shown to reduce the risk of death and hospitalisation in HFrEF patients compared to enalapril therapy alone.1 However, the effects of S/V therapy on cardiopulmonary function and cardiac remodelling have not been fully elucidated. In our observational, prospective and multicentre study, we evaluated the early and late effects of S/V therapy on the functional capacity of patients with HFrEF as assessed by CPET parameters. During follow-up, we observed a significant improvement in the main CPET parameters in the short, medium and long term. At the same time, we demonstrated an improvement in the indexes of LV systolic and diastolic function, in line with previous findings.
Palau et al. observed an increase in VO2 peak and a decrease in the VE/VCO2 slope in a small cohort of HFrEF patients over a mean follow-up of 30 days.15 In our study, conducted in a population of 134 patients with a mean follow-up of 13.7 ± 6.6 months, a significant increase in VO2 peak, predicted VO2, O2 pulse and VO2 was confirmed. A reduction of the VE/VCO2 slope was already observed after 3 months of treatment, persisting during long-term follow-up (24 months). Swank et al. showed that for each 6% increase in VO2 peak there is an 8% reduction in cardiovascular mortality and hospitalisation for HF and a 7% reduction in mortality from all causes.16 Arena et al. observed that patients with a VE/VCO2 slope >34 showed an increase in cardiac mortality at 1 year (survival 83.1% versus 99.2% in those with slope <34; p<0.0001) and an increase in emergency hospitalisation for cardiac causes compared to patients who had a value of this ratio <34.17 Furthermore, numerous pieces of evidence confirm the prognostic importance of the VE/VCO2 slope in patients with HF.18–23 The results of our study show that patients treated with S/V had a significant reduction in VE/VCO2 in the short, medium and long term, regardless of the S/V dosage used.
In a post hoc analysis of the PARADIGM-HF study, Vardeny et al. showed that – even at low doses – S/V leads to clinical benefits compared to enalapril; however, patients taking low doses have a higher risk of primary events.24 In our study, patients taking low doses of S/V had less improvement in oxygen pulse and in VO2/work than those taking high or intermediate doses. It could be argued that these results are linked to the greater fragility of patients treated with lower doses of the drug; the latter, in fact, had lower systolic blood pressure values both at baseline and during the follow-up, higher levels of NT-pro BNP, higher prevalence of NYHA III class, higher doses of furosemide, reduced values of eGFR and higher ratios of VE/VCO2 at baseline.
In a recent study, Guazzi et al. demonstrated that subjects treated with enalapril had higher peak VO2 values and lower VE/VCO2 slope values compared to those treated with placebo.11,25 These results could be due to an improvement in pulmonary alveolar-capillary diffusion mediated by an increase in bradykinins and, consequently, by prostaglandin-mediated vasodilation. These authors observed a statistically significant increase in lung diffusion capacity of carbon monoxide (DLCO) and an inverse relationship between DLCO and VD/VT. The same authors demonstrated that treatment with losartan was associated with a significant increase in the ΔVO2/Δ work ratio (p<0.05 in patients treated with losartan versus enalapril), probably due to improved peripheral muscle perfusion. Furthermore, a synergistic effect between enalapril and losartan on exercise capacity and VO2 peak was shown in patients treated with both drugs compared to patients on monotherapy.11, 25
Accordingly, the present data confirm an improvement in exercise tolerance during follow-up both in terms of VO2/work (Δ = +0.8 ml/beat, p=0.0002) and VO2 at the anaerobic threshold. (Δ = +1 ml/kg/min, p=0.021).
The improvement of exercise tolerance during therapy with S/V is likely because of the combination of the effect of ARBs and neprilysin inhibitors: Valsartan is an angiotensin II type I (AT1) receptors antagonist (that antagonises the effects of angiotensin II, causing vasodilation and volume reduction), while sacubitril is an inhibitor of the enzyme neprilysin (a neutral endopeptidase responsible for the degradation of vasoactive peptides, including natriuretic peptides, bradykinin, and adrenomedullin. By this mechanism, the levels of these substances increase causing vasodilation and natriuresis). As a consequence of these medications’ actions, an amplification of the system of natriuretic peptides and other vasoactive peptides (such as bradykinin, adrenomedullin, endothelin-1, substance P and angiotensin II) is observed.26,27 Therefore, based on the results of our study, it seems that S/V can improve pulmonary diffusion through an increase in bradykinin and can exert a positive effect on muscle efficiency. This results in a clear improvement in exercise tolerance and muscle performance. In particular, the early improvement in VO2 peak could lead to an increase in pulmonary diffusion, and the simultaneous reduction in the VE/VCO2 ratio could lead to a lasting reduction over time in the LV volume overload with consequent reverse remodelling of the LV. Recently published data demonstrate an improvement in LVEF and effects on reverse remodelling of the LV in patients receiving S/V.28,29
An increase in peak ventilatory response was also observed, which could be secondary to the improved cardiac performance and reduced congestion, allowing patients to increase ventilation without increasing the VE/VCO2 slope. In agreement with these data, an improvement in the ejection fraction, plus a reduction in the E/A ratio and in the size of the inferior vena cava after S/V treatment, was confirmed in our study population.
In the subgroup of patients who underwent CMR, the clinical and demographic characteristics were comparable to those of the entire study population. The morpho-volumetric data obtained by CMR confirmed the echocardiographic data. The mass of DE was also quantified in g and in % related to the mass of the LV. In this subgroup of patients, we observed that patients with DE>4.6% reached a minor improvement in cardiorespiratory function parameters.
Recent studies have suggested a possible role of S/V therapy in the remodelling and deposition of fibrotic material at the myocardial level through an activating action on cardiac fibroblasts.8,30 Few data are available regarding ventricular remodelling, and other mechanisms of benefit are unclear, including reduction of myocardial and fibrosis; nonetheless, given that the benefit of S/V appeared to be related to improvement in rates of progressive pump failure or , it is reasonable to hypothesise significant reverse remodelling related to therapy.
Even in patients with ischaemic heart disease, there are data in the literature demonstrating the role of S/V in reducing cardiac remodelling and LV dysfunction.7 Therefore, S/V therapy may reduce cardiac remodelling in ischaemic and non-ischaemic patients. The results of our analysis suggest that, in patients with myocardial fibrosis, the effects of the drug on functional capacity and cardiorespiratory parameters, even if still evident, are reduced in extent. Finally, in the total population multivariate analysis, the main predictors of major cardiovascular events during follow-up were VE/VCO2>34, the presence of ventilatory oscillation and the haemoglobin value. These results are in accordance with the data present in the literature. In the subgroup of patients undergoing CMR, the main prognostic predictors during follow-up were VE/VCO2 and % DE. To date, the present study represents the largest series of HFrEF patients treated with S/V in whom functional capacity has been assessed through CPET parameters with both short- and long-term follow-up. Further studies are needed to better understand the mechanism of action of the drug and its effects on cardiac remodelling.
Study Limitations
The main limitation of this study is the absence of a control group. However, the study was initiated after the publication of the current guidelines of the recommendation for the use of S/V in patients with HFrEF.10 Having demonstrated a significant reduction in mortality compared to enalapril therapy alone, it would not have been ethically correct to deny treatment to the control group. It was also considered methodologically incorrect to select a control group with contraindications to S/V therapy (e.g. patients with arterial systolic hypotension, class IV–V chronic renal failure), because the two populations under examination would have been highly heterogeneous.
In the subgroup of patients undergoing CMR, the limitations of the small size of the sample and lack of an MRI examination during follow-up should be noted. However, these patients underwent primary preventive ICD implantation in the study and were unable to undergo CMR after initiation of S/V treatment. Finally, a further limitation of the study is that patients undergoing CMR presented a recent diagnosis of HF and for this reason they had not yet been implanted with a device.
Conclusion
In patients with HFrEF treated with S/V in the short, medium and long-term follow-up, a significant improvement in cardiovascular, muscle and ventilatory efficiency was demonstrated. The presence of DE in the myocardium influences the response to therapy with S/V, suggesting that myocardial fibrosis might attenuate the effects of the drug on functional capacity and cardiorespiratory parameters. Further studies are eagerly awaited in order to elicit the drug’s mechanism of action and its effects on cardiac remodelling.
Clinical Perspective
- This study aimed to evaluate the cardiopulmonary effects of sacubitril/valsartan (S/V) therapy in patients with heart failure with reduced ejection fraction (HFrEF) and investigating the role of myocardial fibrosis, assessed with cardiac magnetic resonance (CMR), in predicting response to S/V therapy.
- At the end of our study we concluded that:
- The CPET is confirmed as the best evaluating test for the patients’ setting treated with S/V. During the follow-up, by CPET, we were able to evaluate how S/V improved functional capacity in HFrEF patients.
- A percentage delayed enhancement cut-off (<4,6%) can be established as predictive for a better response to therapy.