Heart failure is an umbrella term for a variety of common and serious problems that are often overlooked in clinical practice.1 Two universal definitions of heart failure have recently been proposed; both highlight the central role of congestion in the pathophysiology and presentation of heart failure.2,3 One definition requires symptoms (such as exertional breathlessness) and clinical signs (such as peripheral oedema) of congestion to make a diagnosis of heart failure.2 However, symptoms and signs are late manifestations of disease and lack specificity until they are severe. Indeed, for many patients, symptoms and signs of heart failure go unrecognised until they are so severe that admission to hospital is required.4 Intervening earlier might delay disease progression more effectively.5,6
Higher plasma concentrations of natriuretic peptides reflect increases in intra-cardiac pressures and transmural myocardial wall tension due to cardiac dysfunction; they are associated with an adverse prognosis even in the absence of obvious symptoms. However, natriuretic peptides provide little-to-no information on the aetiology of heart failure.
Imaging, particularly by ultrasound, provides information on cardiac structure and function to guide introduction of appropriate treatments, and can also be used to identify and quantify congestion – both haemodynamic or vascular – and water excess in the tissues of other organs, as we will discuss in this review (Figure 1).7
Echocardiography
Echocardiography is fundamental in the assessment of myocardial structure and function. Through a complex process involving the transmission and subsequent reflection of ultrasound waves, dynamic motion imaging of the heart can be obtained and interpreted in real time via echocardiography. Compared with other complex imaging modalities, echocardiography is widely accessible, free from radiation, relatively affordable and highly versatile; it can provide detailed information on cardiac haemodynamics and valvular function, either at patient’s bedside or in the outpatient setting.8 As a result, it remains the most useful initial imaging diagnostic tool for the vast majority of patients with suspected or diagnosed heart failure.9
The Left Ventricle
The first question that an echocardiographer is asked to answer in a patient with heart failure is usually: “what is the left ventricular ejection fraction?” In its simplest sense, the left ventricular ejection fraction (LVEF) is the percentage of blood that the left ventricle (LV) ejects during systole, relative to end-diastole. It reflects the systolic contractility of the LV and – although influenced by changes in loading conditions (volume status, blood pressure) and underlying cardiac rhythm (for instance AF) – its accurate assessment is pivotal in determining the heart failure ‘phenotype’. This guides eligibility for medical or device therapies, inclusion in clinical trials and the assessment of recovery of function in optimally treated patients. Assessment of LVEF can be qualitative (subjective) or quantitative (objective). Cardiologists and sonographers should strive towards attainment of objective measures of ejection fraction over subjective ‘eyeball’ measurement; the latter is usually the result of poor echocardiographic windows, suboptimal training or insufficient scan time.10 Simpson’s biplane method is the day-to-day standard for LVEF measurement. In cases of poor imaging quality, LV contrast agents can be used to opacify the LV, allowing more accurate interpretation of LVEF.11 Newer methods for assessing LV volumes using 3D applications have improved accuracy compared with cardiac magnetic resonance (CMR) as a gold-standard.12 The use of 3D echocardiography also aids in the interpretation and quantification of valvular disease, such as mitral regurgitation, which is common in patients with heart failure, but it is reliant on good image quality.13
Speckle tracking echocardiography tracks multiple specific regions, or speckles, of the myocardium and can evaluate global or regional longitudinal, circumferential and radial myocardial deformation.14,15 A worsening (less negative/more positive value) global longitudinal strain (GLS), in particular, correlates with increasing natriuretic peptides and might facilitate identification of those with heart failure and preserved LVEF.16 GLS can also be useful in the longitudinal follow-up of patients being treated with cardio-toxic agents, to identify those in whom a change in oncological and/or cardio-protective therapy might be warranted, or in gauging response to therapy for cardiac amyloidosis.11,17
The Left Atrium
Current diagnostic algorithms proposed to identify elevated cardiac filling pressure are time consuming with limited applicability in routine clinical practice. The left atrium (LA) is a thin-walled chamber that acts as a reservoir, conduit and pump for oxygenated blood prior to transport to the muscular, sub-systemic LV. The LA dilates in response to chronic elevations in pressure, which may be due to LV or mitral valve dysfunction or both. A dilated LA is associated with elevated natriuretic peptide levels and with an increased risk of a broad range of cardiovascular events both in patients with heart failure and the general population, particularly AF, a common precipitant of heart failure symptoms and signs.18–21 Transition from dilation to LA dysfunction and failure might lead to poorer prognosis; treatments that decrease LA size might delay onset of heart failure or progression of disease.22–25
The Right Side of the Heart
The failure to control elevated LV filling pressures eventually leads to right ventricular (RV) dysfunction, development of venous hypertension and poorer prognosis.26 In clinical practice, pulmonary artery systolic pressure can be estimated from the peak velocity of the tricuspid regurgitant jet via Doppler. When elevated, it identifies patients with heart failure with more severe symptoms and greater risk of death; however, reliability of this method is questionable in the presence of severe tricuspid regurgitation or substantial RV dysfunction.27 The inferior vena cava (IVC) diameter and its changes with respiratory manoeuvres can be easily assessed in the vast majority of patients with heart failure to estimate fluid status and right atrial (RA) pressure.28 Almost 20% of outpatients with heart failure who are clinically free from signs or symptoms of congestion have a dilated IVC; interestingly, a dilated IVC can be also identified in >10% of those with cardiovascular risk factors only, such as diabetes or hypertension.29,30 An increasing IVC diameter is associated with a larger LA, higher natriuretic peptides and a greater risk of premature cardiovascular events, regardless of LVEF. Conversely, in patients with chronic heart failure, a non-dilated IVC that collapses >50% with inspiration is associated with better prognosis.31 For those who are hospitalised with heart failure, serial assessment of IVC diameter might be used to monitor response to diuretic therapy; for those in whom the IVC remains dilated at discharge, there is a high risk of an early readmission.32 On-going clinical trials are evaluating whether treatment guided by changes in IVC diameter improves management of congestion in patients hospitalised with heart failure (NCT04549701 and NCT03140566).
Jugular Vein Assessment
When assessment of the IVC is difficult, either because not tolerated or limited by the patient’s body habitus, RA pressures or intravascular volume can be estimated by ultrasonic evaluation of the internal jugular vein (IJV). The IJV lies in the carotid sheath, close to the carotid artery and is covered – superficially – by the sternocleidomastoid muscle in the neck. Using a high frequency transducer, the IJV can be easily imaged in every person.
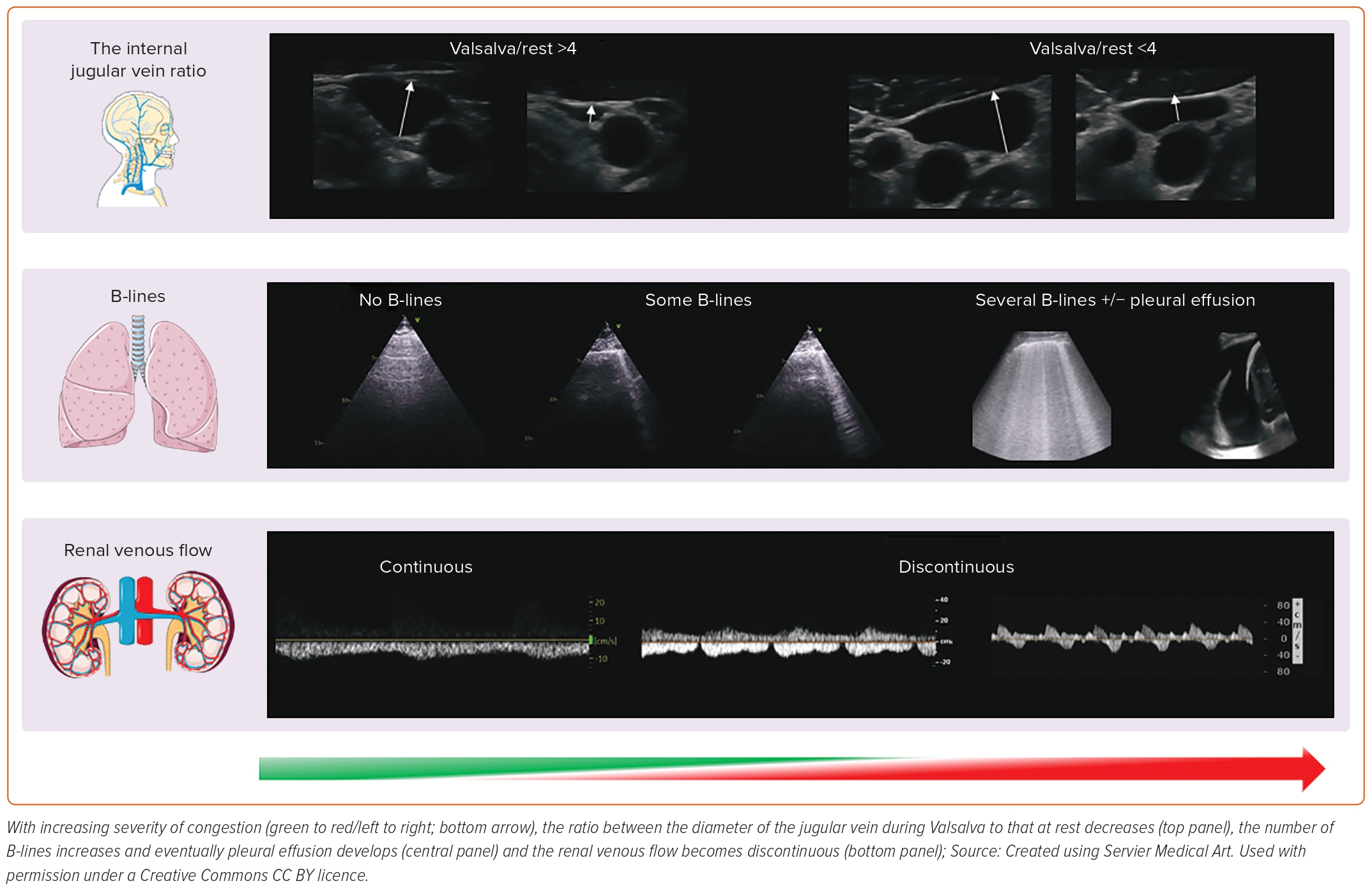
With the patient’s head and neck reclined at 45–60º degrees, in normal haemodynamic conditions, the IJV is almost collapsed; its distension can be provoked by asking the patient to perform a Valsalva manoeuvre, causing a rapid increase in venous pressures. The ratio between the maximal IJV diameter during Valsalva to that at rest or the percentage of cross-sectional area change correlate with natriuretic peptides and RV function.33,34 An already distended jugular vein at rest will only marginally increase in size during a Valsalva: in that case, intravascular congestion is likely to be substantial, as is the risk of hospitalisation or death.35,36
Lung Ultrasound
Frank pulmonary oedema may be triggered by an acute event and usually presents as a symptomatic crisis. However, many patients with heart failure have persistent pulmonary congestion that is not clinically or radiologically obvious in routine clinical practice. Ultrasound is a quick and simple test that can be performed in minutes at the bedside to identify de-aerated segments of the lung, the consequence of which will be vertical artefacts, originating from the pleura that traverse the ultrasound screen, called B-lines. In a breathless patient, multiple (i.e. three or more in a single intercostal space, usually called ‘chest zone’), diffuse (i.e. in more than one chest zone) and bilateral B-lines suggest a diagnosis of heart failure.37 However, other conditions – for instance, adult respiratory distress syndrome or pulmonary fibrosis – may produce lung B-lines and distinguishing among them always requires a global assessment of the patient.38
Different protocols for the assessment of B-lines exist.39 Overall, an increasing number of B-lines is associated with a faster respiratory rate, higher natriuretic peptides and a decreasing BMI; therefore, additional care must be taken when interpreting their number and distribution in obese patients.40,41 Not surprisingly, in patients with either acute or chronic heart failure, an elevated number of B-lines is associated with a greater risk of cardiovascular events and there is accumulating evidence suggesting that it could be a therapeutic target.42,43 The use of lung ultrasound might also facilitate identification of other frequent complications of heart failure, such as pleural effusions, or concurrent lung diseases, such as pneumonia.44
Renal Ultrasound
Heart failure is, in essence, a cardio-renal syndrome: assessment of blood flow within the kidney by ultrasound provides important clinical information. While a few studies suggest that impaired renal arterial flow correlates with higher natriuretic peptides and a poorer outcome, in recent years Doppler assessment of the interlobar veins has received particular attention to estimate central venous pressure (CVP) and intra-parenchymal congestion of the kidneys.45,46 With a normal CVP, there is continuous renal venous flow during the whole cardiac cycle; as renal and intravascular congestion worsen, the flow pattern becomes discontinuous with two (systolic and diastolic; biphasic) or one (diastolic; monophasic) component in more severe cases, suggesting the need for urgent therapeutic action and a poor prognosis.47–49 The use of a convex probe might facilitate the study of the kidney venous flow; however, visualisation of the interlobar veins can be difficult at times, particularly in those with severe renal dysfunction or who are unable to hold their breath for long periods to avoid diaphragmatic movements that interfere with correct renal evaluation by ultrasound.
Other Cardiac Imaging Modalities
Coronary artery disease (CAD) is one of the most common causes of heart failure; its identification is important for risk stratification and – particularly in younger patients – therapeutic decisions.50 Although the STICH trial did not demonstrate that coronary artery bypass graft surgery reduced all-cause mortality in patients with an LVEF ≤35%, extended follow-up suggested a survival benefit, at least for younger patients.51 Excluding CAD in patients with heart failure with a reduced LVEF might also be useful in selecting patients for an ICD.52 Patients with CAD and well-controlled heart failure might also benefit from the addition of rivaroxaban 2.5 mg twice daily and statins.53,54 In order to exclude important CAD, guidelines now recommend that CT coronary angiography may be considered.55 For those who are symptomatic, either due to angina or to ventricular arrhythmias, invasive coronary angiography is currently recommended to assess severity and extent of CAD. CMR imaging can also be used to assess for the presence of significant CAD with stress perfusion imaging, or for evidence of scar from a prior MI with late gadolinium enhancement (LGE).56,57
CMR is also considered the gold standard test for assessing cardiac chamber volumes and, therefore, ejection fraction. CMR is angle-independent and not limited by factors that may have an impact on the quality of an echocardiographic exam such as a poor acoustic window or body habitus.58 CMR has the unique ability among cardiac imaging techniques to provide detailed tissue characterisation, through the use of LGE and non-contrast tissue characterisation with cardiac mapping. Specifically, CMR can identify areas and patterns of myocardial fibrosis or scar, inflammation, fatty infiltration or iron overload, as well as quantifying extracellular volume. CMR’s ability to characterise tissue can be particularly useful in determining the aetiology of heart failure, to improve risk stratification, or to guide individualised treatments in those with suspected sarcoidosis, amyloidosis or other infiltrative or inflammatory cardiomyopathies.56–59 CMR shows that a surprisingly high number of people have myocardial scar consistent with a previous MI despite having no history of such an event. Patients with unrecognised (silent) MI have a similar prognosis to those with a recognised event, suggesting that the gadolinium-enhancement is not merely an artefact.60 Of note (see below), patients with heart failure also have a high prevalence of unrecognised (silent) cerebral infarctions. CMR does have limitations in terms of availability as the scanners are expensive; arrhythmia can reduce the accuracy of image quality and some implantable devices are not CMR conditional. For those with heart failure with cardiac or extracardiac red flags of transthyretin amyloidosis, 99mTc-DPD scintigraphy has a very high specificity and positive predictive diagnostic value for diagnosis.61 An endomyocardial biopsy is usually reserved for selected cases, even in those with myocarditis presenting as heart failure.62
Other Organs
The Bowel
Heart failure may be associated with loss of appetite that – combined with hepatic and intestinal congestion – may lead to iron malabsorption, malnutrition and cachexia.63 There is some evidence that patients with chronic heart failure have increased colonic wall thickness, perhaps reflecting oedema and reduced intestinal blood flow. This, in turn, may result in changes in the gut microbiota, triggering a systemic inflammatory response that may accelerate heart failure progression and death.64,65 Bowel-wall thickness can be assessed by ultrasound and, when increased, is associated with greater congestion and poorer outcomes in patients with heart failure.63,66
The Brain
The relationship between heart and brain dysfunction is complex. Many patients with heart failure report a variety of neurological symptoms, ranging from cognitive impairment and loss of attention to anxiety and depression.67,68 Advanced age and hypoperfusion secondary to a reduced cardiac output or hypotension and atherosclerotic disease of the cerebral vessels might be key drivers of degenerative brain changes associated with heart failure.69 However, micro- or macroembolic events, in the context of atherosclerotic disease in the carotids or an intracardiac thrombus, and/or the presence of other comorbidities common in heart failure such as hypertension, diabetes or AF, might also contribute to and accelerate brain damage. MRI scans suggest that patients with heart failure have several structural cerebral abnormalities, including white matter hyper-intensities, lacunar and cortical infarcts and cortical atrophy, even in the absence of a prior history of neurological symptoms or disease, similar to the high prevalence of silent MI noted above.70 In patients with heart failure, an inverse correlation has been reported between the density of cerebral grey matter in the hippocampus – a region implicated in development of cognitive dysfunction and memory loss – and N-terminal pro-B-type natriuretic peptide concentration; however, for those with less advanced heart failure, progression of hippocampal atrophy over time is minimal and perhaps not different from that expected due to ageing.71–73
Skeletal Muscle
A loss of muscle mass, quality and/or strength – sarcopaenia – occurs in patients with advanced heart failure but, as with so many other aspects of heart failure, its presence and severity often go unrecognised until it is extreme and the patient is overtly cachectic.74 Sarcopaenia, even when not clinically obvious, is associated with adverse outcomes. In clinical practice and research, several imaging methods are available to quantify muscle mass. MRI provides detailed information on muscle quantity and is a non-invasive gold standard tool for research in this setting but is not suitable for clinical use on a large scale due to high costs and limited access.75 Dual-energy X-ray absorptiometry (DEXA) and bioelectrical impedance analysis (BIA) are widely available, affordable and easy to use in an ambulatory setting even by non-medical personnel.76 Although there is a strong correlation between muscle mass measured with these two techniques (correlation coefficient >0.9), some studies suggest that BIA might produce higher readings for muscle mass compared with DEXA. In patients with heart failure, muscle mass measured with both techniques is only weakly associated with age or biomarkers of cardiac stress or inflammation but more closely related to other measures of body size, such as BMI or waist or hip circumference.77
Conclusion
Modern imaging methods enable congestion – both haemodynamic/vascular and in tissues – to be identified and quantified objectively. Patients with cardiac dysfunction who have evidence of congestion on imaging are at increased risk of disease progression, heart failure decompensation and death, even if their symptoms and clinical signs appear adequately controlled. Whether early identification and treatment of congestion will improve outcomes is controversial, but accumulating evidence suggests that this might be the case.