Chronic heart failure (CHF) is a progressive disorder characterised by elevated cardiac filling pressures, reduced cardiac output and decreased oxygen delivery to the tissues.1 Activation of the sympathetic nervous system (SNS), along with activation of the renin–angiotensin–aldosterone system (RAAS), plays a fundamental role in the pathophysiology of CHF syndrome.2–5 Early in the course of heart failure (HF) development, the neuro-endocrine system is activated and maintains haemodynamic stability and cardiac output, but over time these compensating mechanisms lead to deterioration of cardiovascular function through several pathways.6 Thus, inhibition of SNS by beta-blockers and RAAS by angiotensin converting enzyme inhibitors, angiotensin receptor blockers and mineralocorticoid receptor antagonists has become the current standard pharmacological treatment for CHF. Despite the widespread use of these drugs, CHF patients still remain at high risk of death and worsening HF, possibly because of suboptimal drug therapy management.
There is growing clinical evidence that more than half of patients with CHF who are on beta-blockers have inadequately controlled heart rate (HR)7–11 and a substantial proportion of patients do not tolerate the target doses of beta-blockers used in the large clinical trials.8 Moreover, further up-titration of beta-blockers is not achievable in many patients.12 This is of concern since elevated HR is associated with an increased incidence of cardiovascular events in patients with CHF.13–16 High resting HR has been found to be a predictor for clinical outcomes17 and total and cardiovascular mortality independent of other risk factors in patients with coronary artery disease18 and in the general population, as well as in CHF patients.19 There is therefore a need for further strategies to reduce HR in CHF patients. Within this framework, clinical data support the addition of ivabradine to betablocker therapy. This brief review aims to summarise clinical evidence supporting the combined use of beta-blockers and ivabradine in patients suffering from systolic CHF.
SNS Activation and Beta-blocker Therapy in CHF
The left ventricle ‘remodelling’ process, resulting in a progressive enlargement of the left ventricle and decline in contractility – one measure of which is a reduced ejection fraction (EF) – characterises CHF with systolic dysfunction.20,21 Continued SNS activation over time results in myocardial injury and in systemic effects that are detrimental for the blood vessels, kidneys and muscles. Together with RAAS activation, this creates a pathophysiological cycle responsible for worsening CHF syndrome and death.20,21 The altered haemodynamic homeostasis of CHF patients is associated with an increased HR, which carries a negative prognosis;22–24 whereas the beneficial effect of beta-blockers has been linked to their HR-lowering effect.14–25 However, as previously mentioned, beta-blockers are often underused in clinical practice, are seldom prescribed at the doses proven to reduce events,26–29 and their up-titration in response to persistently elevated HR can be associated with an increased risk of adverse reactions.12 A non beta-blockade approach to HR reduction has recently become available30,31 following discovery of the If current that modulates the slope of spontaneous diastolic depolarisation of the sino-atrial node: namely, ivabradine.
Use of Ivabradine in Heart Failure
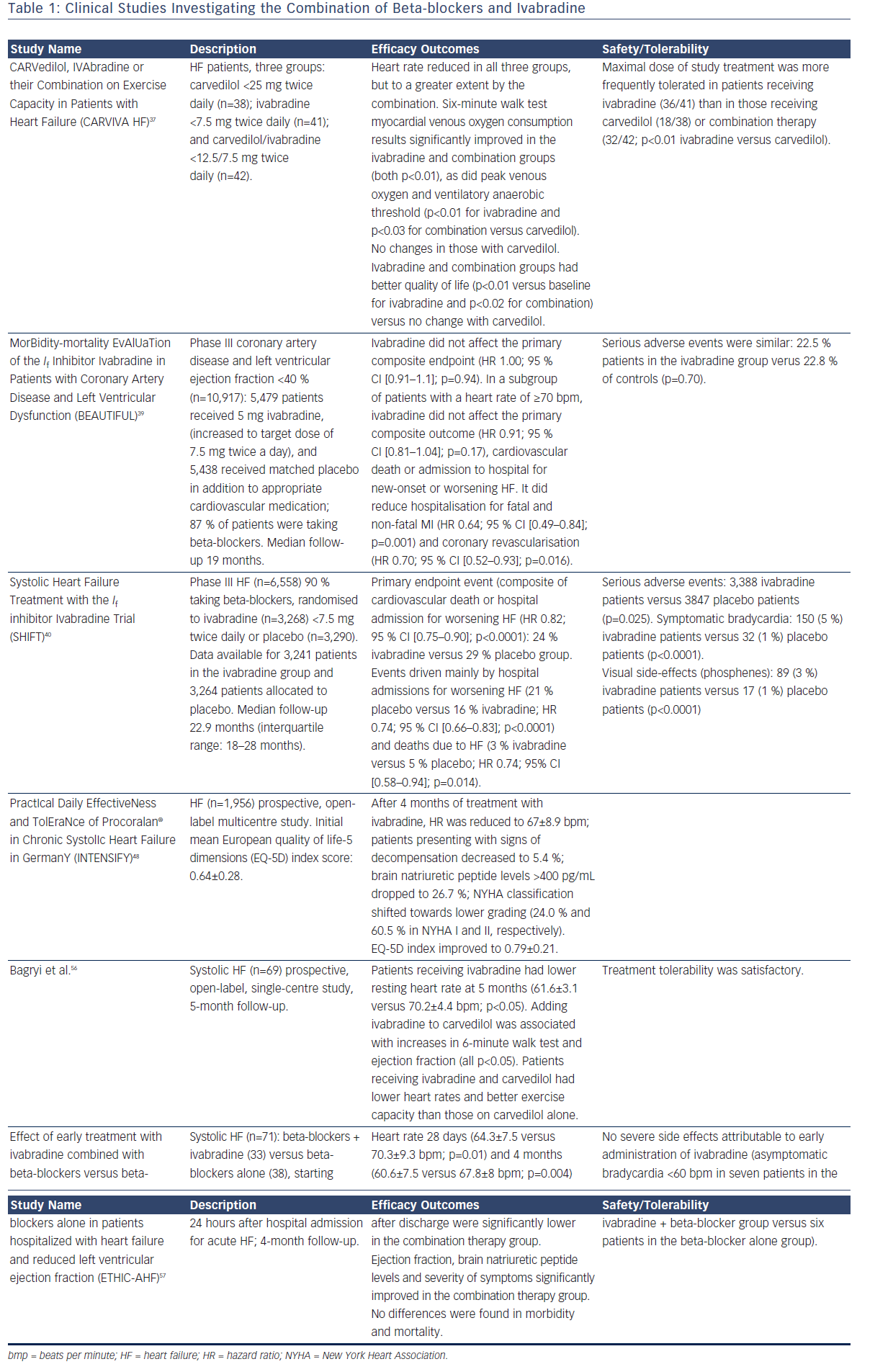
Ivabradine (Procolaran®, Servier) selectively and specifically inhibits the If current in the sino-atrial node, reducing HR without affecting the autonomic nervous system (see Figure 1).32–34 The effectiveness of ivabradine in CHF has been tested in the Systolic Heart Failure Treatment with the If inhibitor Ivabradine Trial (SHIFT)35 in which 6,558 patients with CHF on stable background therapy, including beta-blockers, and a HR ≥70 beats per minute (bpm) with sinus rhythm were randomised to ivabradine (up to 7.5 mg twice daily) or placebo. At the median follow-up of 22.9 months, the results indicated improved clinical outcomes: 18 % reduction in the primary composite endpoint of cardiovascular death or hospitalisation for worsening HF, 26 % reduction in hospitalisation for worsening HF and 26 % reduction in pump failure death in the ivabradine group. Since the majority of patients in SHIFT were taking beta-blockers, it was hypothesised that the combination of beta-blockers plus ivabradine rather than the dose of beta-blocker was relevant to these findings. A subanalysis of SHIFT appeared to confirm this hypothesis, by showing that the combination of drugs rather than the dose of beta-blockers was important in improving the primary endpoints of cardiovascular death and hospitalisation.36 A further study concluded that the combination of beta-blockers plus ivabradine resulted in improved outcomes regardless of the individual beta-blocker prescribed.37
A number of clinical studies have evaluated the use of ivabradine in combination with beta-blockers (Table 1). The first large randomised controlled study of ivabradine was the MorBidity-mortality EvAlUaTion of the If Inhibitor Ivabradine in Patients with Coronary Artery Disease and Left Ventricular Dysfunction (BEAUTIFUL) trial38 in which patients (n=10,917) with stable coronary artery disease and an EF <40 % were randomised to ivabradine 7.5 mg twice daily or placebo. Most patients (87 %) were receiving beta-blockers in addition to the study drug. After a median of 19 months, no significant difference was found between ivabradine and placebo in terms of the primary composite endpoints (cardiovascular death, hospitalisation for myocardial infarction and worsening HF). However, ivabradine reduced hospitalisation for myocardial infarction and coronary revascularisation in patients with a HR >70 bpm by 36 % (p=0.001), suggesting that the lowering of raised HR may be associated with improved outcomes. Importantly, the study also showed that the combination of a beta-blocker and ivabradine was well tolerated.
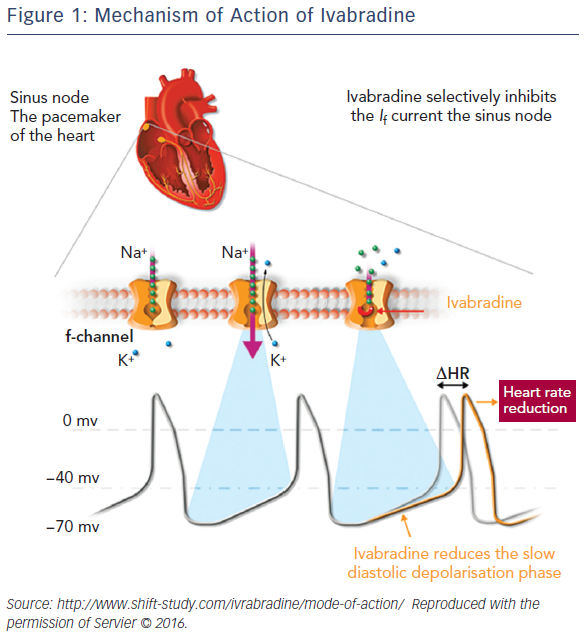
In SHIFT,35 patients with symptomatic CHF had a higher baseline HR and a greater HR reduction due to ivabradine than in the BEAUTIFUL trial. In this study, patients (n=6,558) with CHF on stable background therapy were randomised to ivabradine (up to 7.5 mg twice daily) or placebo. At the median follow-up of 22.9 months, data were available for 3,241 patients in the ivabradine group and 3,264 patients in the placebo group. Use of ivabradine was associated with an 18 % reduction in the primary composite endpoint of cardiovascular death or hospitalisation for worsening HF: 24 % of patients in the ivabradine group and 29 % of those taking placebo had a primary endpoint event (hazard ratio (HR) 0.82; 95 % CI [0.75–0.90]; p<0.0001; Figure 2). The effects were driven mainly by hospital admissions for worsening HF (21 % placebo versus 16 % ivabradine; HR 0.74, 95% CI [0.66–0.83]; p<0.0001) and deaths due to HF (5 % versus 3 %; HR 0.74; 95 % CI [0.58–0.94]; p=0.014). The risk of cardiovascular outcomes increased with HR, and every 5-bpm increase in baseline HR was associated with a 16 % increase in the risk of primary outcome in the placebo arm.35 This is in agreement with a meta-analysis by McAlister et al. indicating that, in CHF patients, a reduction of 5 bpm with beta-blocker treatment was associated with an 18 % reduction in the risk of death.39 An analysis of the SHIFT data found that in the ivabradine group there was a direct association between HR achieved at 28 days and subsequent cardiac outcomes. Patients receiving treatment who reached a target HR below 60 bpm at 28 days had the lowest event rate compared with patients with higher HRs (event rate 17.4 %; 95 % CI [15.3–19.6]), suggesting that resting HR is a powerful predictor of outcomes in CHF.13
Since the majority of patients in SHIFT (90 %) were taking beta-blockers, researchers hypothesised that the combination of beta-blockers plus ivabradine was important. A substudy of SHIFT to assess the impact of background beta-blocker dose on response to ivabradine found that the combination rather than the dose was important, and that the primary endpoint and HF hospitalisations were significantly reduced by ivabradine in all subgroups with <50 % of target beta-blocker dose, including patients not taking beta-blockers (p=0.012).36 A further study concluded that the combination of beta-blockers plus ivabradine resulted in improved outcomes regardless of the individual beta-blocker prescribed.37
Pathophysiological Mechanisms Underlying the Combined Use of Beta-blockers and Ivabradine
The rationale for combining beta-blockers and ivabradine is that their actions at heart level are synergic and not limited to sinus node rate; whereas beta-blockers have several other target points that are beneficial in CHF syndrome. The randomised CARVedilol, IVAbradine or their Combination on Exercise Capacity in Patients with Heart Failure (CARVIVA-HF) study40 found that ivabradine alone or in combination with carvedilol was more effective than carvedilol alone in improving exercise tolerance and quality of life (QoL) in CHF patients. A subanalysis of SHIFT41 also found that HR reduction with ivabradine was associated with improved QoL. This finding was consolidated by data from the prospective, open-label multicentre PractIcal Daily EffectiveNess and TolEraNce of Procoralan® in Chronic SystolIc Heart Failure in GermanY (INTENSIFY) study.42
The beneficial effects of beta-blockers may only in part be related to HR reduction; their protective action against the deleterious effects of excessive sympathetic activity on the heart and other organs and their humoral mechanisms may significantly contribute to the benefits of this class of drugs. Similarly, it has been suggested that HR-independent mechanisms could contribute to the additional beneficial effects associated with ivabradine treatment.43
Haemodynamic Mechanisms
HR reduction can increase the duration of diastole44,45 and therefore improve myocardial perfusion. Beta-blockers reduce HR and prolong diastolic duration, but they also impair isovolumic ventricular relaxation, offsetting part of this benefit in terms of the diastolic pressure–time integral.46 Beta-blockers also increase alpha-adrenergic coronary vasoconstriction. Ivabradine protects isovolumic ventricular relaxation and does not offset the benefit in terms of coronary blood flow46 because ivabradine does not increase alpha-adrenergic coronary vasoconstriction, as is typically seen with beta-blockers.47 This explains why, for the same level of HR reduction, the increase in diastolic time and the perfusion duration and volume are greater with ivabradine than with beta-blockers.4 Treatment with beta-blockers plus ivabradine therefore improves myocardial perfusion by these mechanisms, both at rest and during exercise.45
In addition to this, ivabradine administration has been shown to significantly increase stroke volume in patients with severe CHF.48 The increase in stroke volume caused by ivabradine is of clinical relevance as beta-blockers reduce stroke volume during initiation, the first months of treatment and up-titration. This effect of betablockade could be compensated for by prescribing ivabradine with lower initial doses of beta-blockers. Ivabradine also reduces left ventricular (LV) end-diastolic pressure, unlike beta-blockers, with this effect being still present when ivabradine is co-prescribed with a beta-blocker, resulting in increased stroke volume and maintenance of cardiac output.48,49
Left Ventricular Structure and Function
In addition to the haemodynamic mechanisms reported above, ivabradine slows the progressive modification of LV structure in the 2–3 months after the initiation of therapy, which contributes to the improvement in cardiac function.49–51 Ivabradine increases vascular compliance, thus reducing LV load, and this effect is related to beneficial outcomes in ivabradine-treated patients. Like beta-blockers, treatment with ivabradine significantly lowers RAAS activation compared with placebo. Lower RAAS activation results in improved renal and vascular pressures, and in a decrease in cardiac wall stress, thereby preventing worsening cardiac fibrosis and cardiac remodelling.
Cardiac remodelling plays a crucial role in the pathophysiology of CHF and also affects the prognosis of this patient population.52 Ivabradine has been reported to induce reverse remodelling in patients with New York Heart Association Functional class II and IV HF, including modifications of LV structure (i.e. decreased in LV end-systolic and end-diastolic volumes), that have been observed after just 3 months of therapy,50,51 being accompanied by a 2.7 % increase in LVEF. A reduction in cardiac collagen53,54 and fibrosis have been also reported in animal models of HF. Another SHIFT substudy found that ivabradine reverses cardiac remodelling in patients with CHF and LV systolic dysfunction, and this effect was independent of beta-blocker use.51 Taken together, these results support the role of ivabradine in protecting LV structure and function.34
Safety Issues Associated with Combined Ivabradine and Beta-blocker Therapy
Studies to date indicate that ivabradine is well tolerated in combination with beta-blockers. In the SHIFT35 there was a lower incidence of serious adverse events in the ivabradine versus placebo group. In total, 21 % of patients on ivabradine discontinued treatment compared to 19 % of patients on placebo (p=0.017). Bradycardia was more frequent with ivabradine than with placebo (5 % versus 1 %, p<0.0001 respectively). Visual luminous phenomena (phosphenes) were reported in 3 % of patients in the ivabradine group.35 Adverse events were not influenced by beta-blocker dosage.37
In the BEAUTIFUL trial38 no additional safety concerns were identified in patients taking beta-blockers plus ivabradine. The incidence of serious adverse events in the ivabradine and placebo groups was similar (22.5 % versus 22.8 %; p=0.70). There was, however, a higher incidence of bradycardia (including asymptomatic bradycardia) in the ivabradine group than in the placebo group (13 % versus 2 %).
Current Ivabradine Use
Currently, ivabradine can be given early in hospitalisation, and can be initiated at the same time as beta-blockers.55,56 It is recommended that treatment commence with the administration of 5 mg ivabradine twice daily. After 2 weeks, the resting HR should be checked. If it exceeds 60 bpm, the dose should be raised to 7.5 mg twice daily. At a resting HR of 50–60 bpm, the dose can be maintained at 5 mg twice daily, and if HR is below 50 bpm it should be reduced to 2.5 mg twice daily.6 HR should be regularly checked throughout treatment and the dose adjusted accordingly. If HR remains below 50 bpm despite dose reduction, treatment must be discontinued. In patients aged 75 years or more, a lower starting dose should be considered (2.5 mg twice daily, i.e. half a 5 mg tablet twice daily) before up-titration if necessary. Importantly, no dose adjustment is needed in patients with hepatic or renal impairment.57
Practical guidance on the use of ivabradine in CHF is provided in an addendum to the 2016 European Society of Cardiology (ESC) Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure (see Table 2).58
Discussion
Current ESC guidelines59 on CHF recommend the use of ivabradine in symptomatic patients with LVEF ≤35 % who are in sinus rhythm and have a resting heart rate ≥70 bpm despite treatment with an evidencebased dose of beta-blocker (or maximum tolerated dose below that or those who are unable to tolerate or have contraindications to a beta-blocker), angiotensin converting enzyme inhibitor, angiotensin receptor blocker and mineralocorticoid receptor antagonist. The US Food and Drug Administration has recommended similar indications for ivabradine.60
It should be recalled that in SHIFT only around a quarter of patients achieved the recommended ESC target dose, and around half achieved at least 50 % of the target dose.35 This reflects current clinical practice.35,61 SHIFT has provided evidence for additional HR lowering with ivabradine for patients in sinus rhythm who are receiving betablockers. Ivabradine is easier to use than beta-blockers and is better tolerated. The benefit provided by ivabradine was similar in the small subgroup of SHIFT that did not receive a beta-blocker to that observed in the overall population,36 raising the possibility that combining ivabradine with suboptimal doses of beta-blockers may be a better strategy than uptitrating beta-blockers to an optimal dose.
One study found that the use of beta-blockers and resting HR were independent predictors of prognosis but beta-blocker dose was not.62 Thus, it may be hypothesised that achieving a HR within the target range may be a more appropriate therapeutic goal than optimising beta-blocker dose in patients with CHF. In SHIFT, patients with the lowest risk reached a HR <60 bpm; therefore it might be reasonable, at present, to recommend this target in daily practice. There is, however, no direct evidence for this. Further trials are clearly needed before first-line use of ivabradine is recommended in patients other than those for whom beta-blockers are contraindicated.
Conclusion
In this review we have reported consistent data suggesting it is possible to safety extend the use of ivabradine plus beta-blocker therapy in patients with CHF, even in less advanced stages of the disease. This combined therapy could favour lower beta-blocker doses, facilitate up-titration for the achievement of target HR, and avoid the possible dose-dependent adverse events related to their use.