Type 2 diabetes (T2D) is becoming increasingly prevalent across the world. Its cardiovascular complications are major causes of mortality and use of medical resources.1 Prevention of cardiovascular diseases is, therefore, an important goal of the treatment of T2D. Metformin is the first-line therapy, according to the European Society of Cardiology, the American Diabetes Association (ADA) and the International Diabetes Federation.2–4 After metformin, three new antidiabetic drug classes have emerged as second-line therapy options.
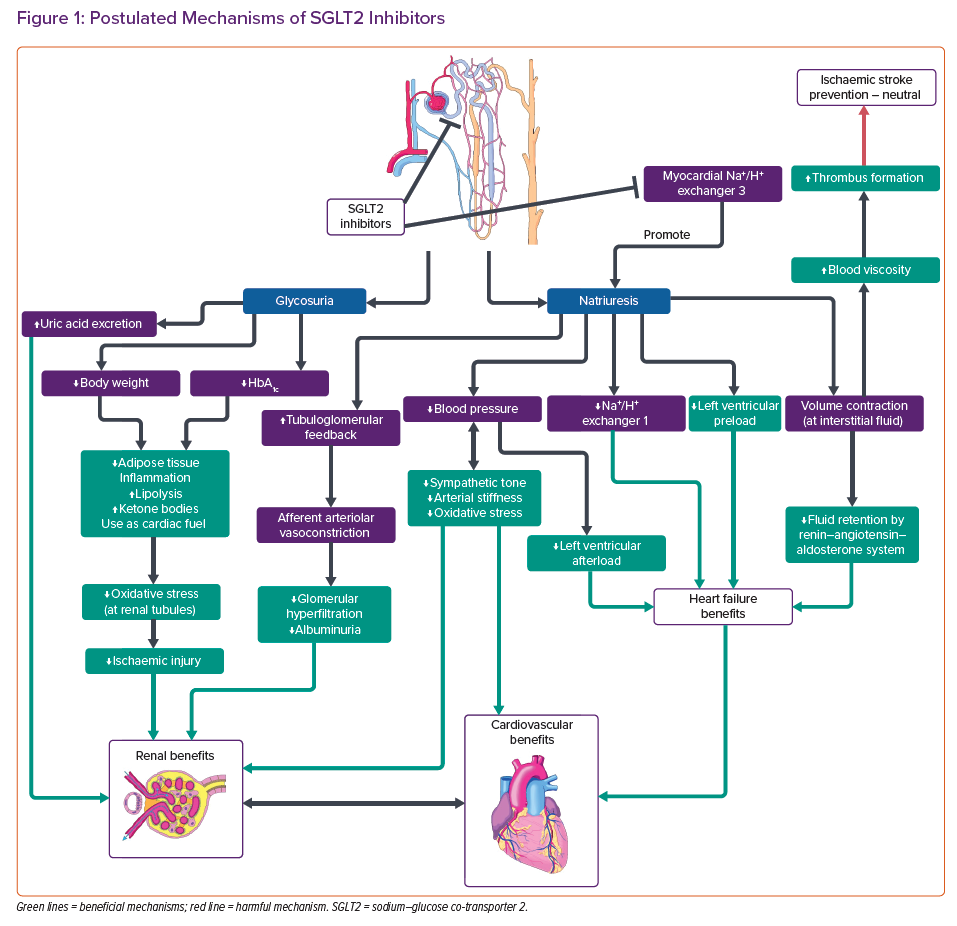
Sodium–glucose co-transporter 2 (SGLT2) inhibitors exert hypoglycaemic effects by inhibiting glucose reabsorption at the proximal convoluted tubules, causing glycosuria, natriuresis and volume contraction (Figure 1).5
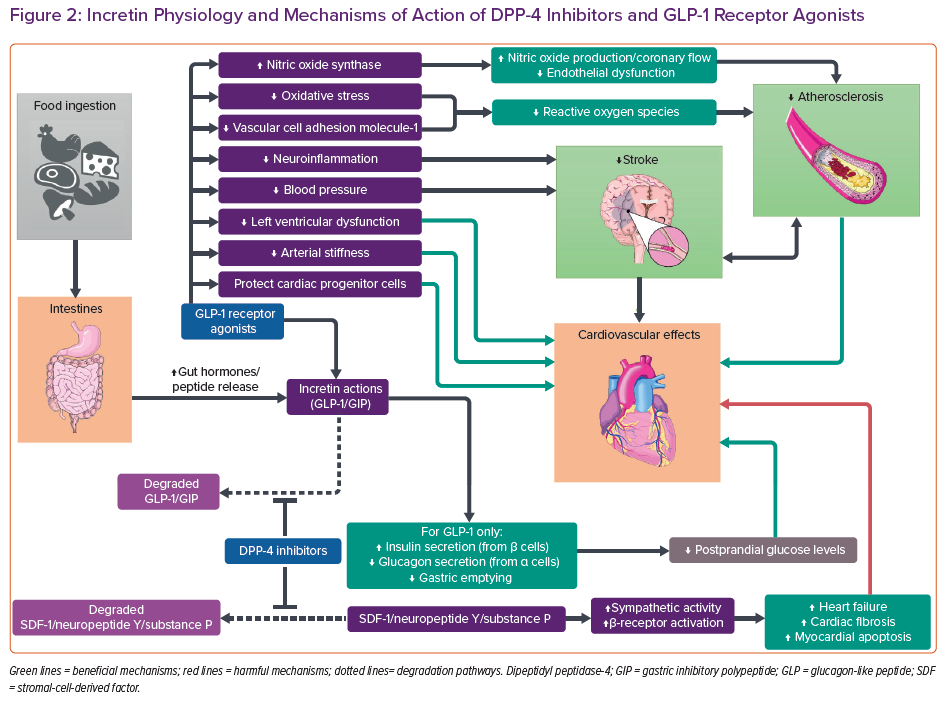
Besides SGLT2 inhibition, incretin-based therapies have also been used in recent years for the treatment of T2D (Figure 2). Incretins are gut-derived hormones that send a signal to the pancreas after the ingestion of food.6,7 There are two main incretin hormones: glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP, also known as glucose-dependent insulinotropic polypeptide). Both are secreted by enteroendocrine cells in the intestines and stimulate pancreatic beta-cells to secrete insulin.7 GLP-1 additionally inhibits glucagon release by pancreatic alpha-cells and delays gastric emptying.6 GIP, on the other hand, also stimulates glucagon production, yet fails to stimulate insulin secretion in people with diabetes.7 Therefore, it has not been developed as a therapeutic agent. The plasma half-life of GLP-1 is short, so GLP-1 receptor agonists (RAs) have modifications in the peptide to prolong half-life.6 As these incretin hormones are degraded by dipeptidyl peptidase-4 (DPP-4), DPP-4 inhibitors can amplify their pharmacological actions.8 Both of these two incretin-based therapies improve postprandial glucose control.6,8
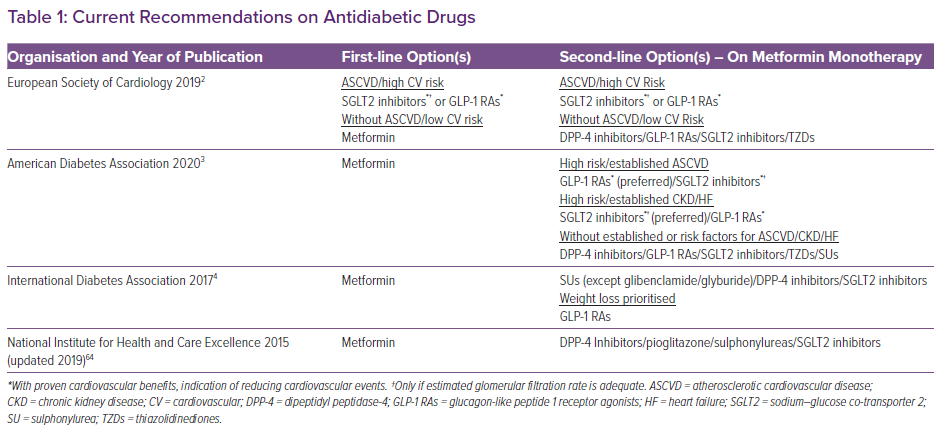
Among the three drug classes, the preferred second-line treatment remains unclear (Table 1). However, the thiazolidinediones are not favoured as second-line drugs. Indeed, pioglitazone-induced heart failure (HF) and the withdrawal of rosiglitazone because of cardiovascular concerns eventually led to a change in the policy of regulatory authorities.9,10
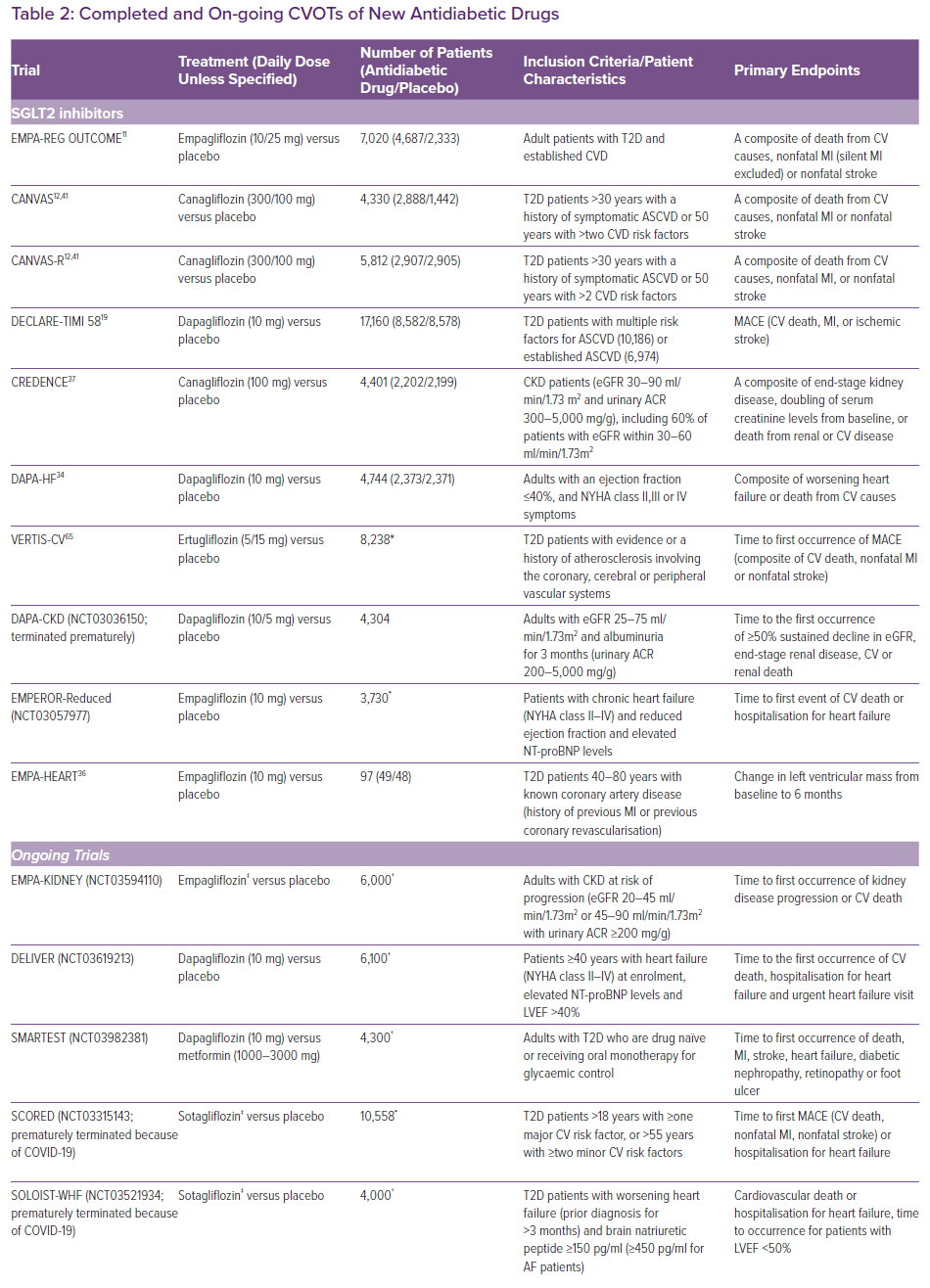
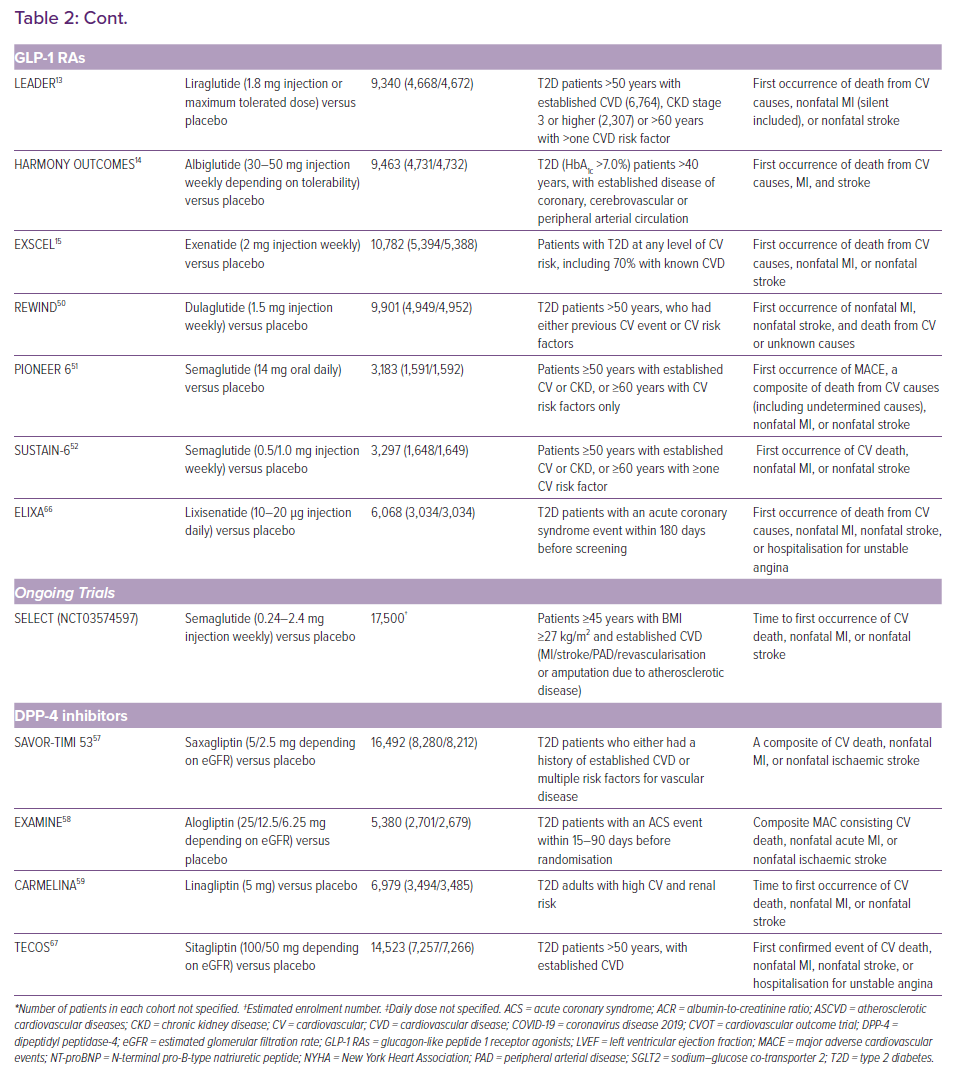
The US Food and Drug Administration and the European Medicines Agency now require all new antidiabetic drugs to undergo large cardiovascular outcome trials (CVOTs) to confirm cardiovascular safety and benefits. As a result of this requirement, multiple CVOTs have been published in recent years (Table 2). Some trials have shown cardiovascular benefits for GLP-1 RAs and SGLT2 inhibitors, which have been confirmed in meta-analyses.11-18 However, their effects on particular outcomes remain inconsistent in trials.15,19,20 This may be a result of limitations in statistical power and differences in patient characteristics and the drugs used. In the absence of adequately powered head-to-head trials, superiority amongst the three antidiabetic drug classes cannot be established.
Network meta-analyses (NMAs) can evaluate comparative risks or benefits using indirect evidence. Our 2019 NMA included 14 trials and a total of 121,047 patients.17 First, we found that both SGLT2 inhibitors and GLP-1 RAs significantly reduced major adverse cardiovascular events (MACE), hospitalisation for HF and renal composite outcome compared to placebo. Second, SGLT2 inhibitors were shown to have the greatest cardiovascular and all-cause mortality benefits amongst all three new antidiabetic drug classes. Third, the GLP-1 RA class was the only one that showed reductions in nonfatal stroke events. Finally, the risks of cardiovascular and renal outcomes in DPP-4 inhibitors were found to be neutral when compared to placebo and inferior to the other two drug classes.
SGLT2 Inhibitors
The EMPA-REG OUTCOME and CANVAS studies have shown favourable all-cause mortality and cardiovascular outcomes.11,12 Meta-analyses of CVOTs confirmed the cardiovascular benefits.17,18 Three studies from landmark CVOTs further stratified patients according to baseline characteristics.21,22,23 The benefits of SGLT2 inhibitors were more apparent in patients with a history of HF and reduced ejection fraction.22,23 However, baseline risk was higher in this group of patients, possibly accounting for higher absolute risk reductions in cardiovascular outcomes. The DECLARE-TIMI 58 trial enrolled a majority of patients who did not have established atherosclerotic cardiovascular disease (ASCVD), and did not show significant reduction in MACE.19 This may imply the cardioprotection offered by SGLT2 inhibitors is less evident in such a group of patients .18 However, analysis from the EMPA-REG OUTCOME trial reported consistent benefits regardless of baseline risks and prior history.21 Neither of these trials was designed to investigate HF outcomes, but they illustrated the value in identifying patient subgroups that benefit most from SGLT2 inhibitor treatment.
SGLT2 inhibitors are postulated to induce selective volume contraction.24 Thus, there is a selective reduction of interstitial fluid in contrast to traditional diuretics.24 Therefore, intravascular volume depletion and fluid retention by the renin–angiotensin–aldosterone system (RAAS) in the long run are limited.25 SGLT2 inhibitors also inhibit myocardial Na+/H+ exchanger (NHE) 1,5 which has been hypothesised to attenuate cardiac hypertrophy and HF development.26 Natriuresis is reported to improve left ventricular (LV) preload conditions, which could be potentiated by additional NHE-3 inhibition at the proximal tubules. 5,27 Furthermore, SGLT2 inhibitors reduce sympathetic tone and blood pressure (BP), which would improve afterload. 5,28,29 SGLT2 inhibitors are therefore particularly useful for diabetic patients with HF.3 Moreover, attenuation in endothelial dysfunction and arterial stiffness by reducing oxidative stress has also been reported, bringing potential benefits in vascular diseases.29,30 Dapagliflozin and empagliflozin have also shown anti-fibrotic effects in rats and human cardiac fibroblasts.31,32 Importantly, SGLT2 inhibitors induce weight loss and increase HDL levels in trials.11,12,26 They also increase lipolysis and reduce inflammation in adipose tissues.33 The switch from utilisation of glucose to ketones is also believed to be beneficial, especially for cardiac metabolism.5 All of these mechanisms may help to explain the cardiovascular benefits observed.11,12
The DAPA-HF and the EMPEROR-Reduced (NCT03057977) trials showed statistically significant reductions in HF hospitalisation, regardless of history of diabetes.34 However, the change in mortality did not reach statistical significance in EMPEROR-Reduced.35 The cardioprotective mechanisms independent of glucose lowering are still not fully understood. The EMPA-HEART trial showed reduction in LV mass and systolic BP in HF with reduced ejection fraction (HFrEF) patients, although the effects on N-terminal pro-B-type natriuretic peptide (NT-proBNP) was insignificant.36 The EMPERIAL trials (NCT03448419 and NCT03448406) reported no significant improvements in exercise capacity measured by 6-minute walking distance. Improvements in quality of life scores were found in patients with HFrEF but not in those with HF with preserved ejection fraction. Results from on-going EMPA-KIDNEY (NCT03594110), DELIVER (NCT0319213), SMARTEST (NCT03982381), as well as the recently terminated SCORED (NCT03315143) and SOLOIST-WHF (NCT03521934) trials will further elucidate the cardioprotective effects and identify patients who may benefit most from treatment with SGLT2 inhibitors.
SGLT2 inhibitors have shown clear-cut renal benefits in the CREDENCE and DAPA-CKD (NCT03036150) trials, which have also been confirmed in meta-analyses.17,37 The natriuresis induced by SGLT2 inhibitors stimulates tubuloglomerular feedback and vasoconstriction in afferent arterioles, thus reducing glomerular hyperfiltration and albuminuria.26,38,39 Concomitant use of RAAS inhibitors is reported to have synergistic effects on renal tubules, slowing down the progression of chronic kidney disease (CKD).40 The aforementioned shift in metabolic energetics is believed to inhibit oxidative stress and ischaemic injury, not only in myocardium but also in renal tubules.29,38 Reduction in BP and body weight as well as increase in uric acid excretion are all postulated as renoprotective mechanisms.39
The benefits of SGLT2 inhibitors are not always apparent. The effect of SGLT2 inhibitors on stroke events appears to be neutral or even slightly harmful. The EMPA-REG OUTCOME trial detected slight elevations in nonfatal stroke events.11 In a subgroup analysis of the CANVAS trial, the results showed protective effects against haemorrhagic stroke, yet neutral effects on ischaemic stroke (HR 0.95; 95% CI [0.74–1.22]).41 BP reduction may account for the prevention of haemorrhagic stroke.42 However, elevated haematocrit was also observed in the EMPA-REG OUTCOME trial, which might predispose to ischaemic strokes.11,42,43 Although haemoconcentration improves cardiovascular outcomes, the higher blood viscosity may trigger thrombus formation.27,42–44 Although weight loss should protect against ischaemic stroke, compensatory changes may limit weight loss in the long term.33 Although a harmful effect on stroke has not been confirmed, the protective effect on haemorrhagic stroke may mask the effect on ischaemia if both types of stroke are included as a composite endpoint.16–18 Population-wide observational studies may help to evaluate the long-term risk of ischaemic strokes during SGLT2 inhibitor therapy.
Overall, SGLT2 inhibitors have demonstrated the most favourable cardiovascular and all-cause mortality outcomes. Indirect evidence showed overall superiority over DPP-4 inhibitors, which appears to be a class effect.16,17 However, superiority between GLP-1 RAs and SGLT2 inhibitors varies across different outcomes. SGLT2 inhibitors are superior in terms of mortality outcomes, yet protection against MACE was similar among GLP-1 RAs and SGLT2 inhibitors.16,17 Also, mortality benefits did not reach statistical significance in the CREDENCE and DECLARE-TIMI 58 trials.19,37 The CREDENCE trial was prematurely terminated because of overwhelming renal and cardiovascular benefits. A shortened follow-up period limited the power of the study to detect changes in mortality, if present. Besides the CREDENCE trial, other non-empagliflozin trials also failed to show significant changes.12,19 Trials of non-empagliflozin SGLT2 inhibitors investigating mortality endpoints are needed to fill in the evidence gap. The combination of RAAS and SGLT2 inhibitors should be advocated as concomitant inhibition appears to achieve better cardiovascular and renal outcomes.40
GLP-1 Receptor Agonists
The LEADER, HARMONY OUTCOMES and EXSCEL trials demonstrated the cardiovascular safety of GLP-1 RAs.13–15 Meta-analyses have reported favourable cardiovascular safety profiles for GLP-1 RAs, especially in MACE and nonfatal stroke events.16,17,45 Improvements in the composite kidney outcome were also detected.45 However, the benefits were less clear-cut in comparison with SGLT2 inhibitors and somewhat conflicting across trials. Discrepancies in mortality-related outcomes could be due to differences in follow-up periods and study population. Intraclass differences and variations in pharmacokinetics are also possible explanations.
Liraglutide, a long-acting GLP-1 RA, yielded favourable results in the LEADER trial.13 In addition to glycaemic control, slowing of atherosclerosis and anti-inflammatory actions also account for the benefits of GLP-1 agonism. The interaction between GLP-1 RAs and cardiac GLP-1 receptors has been suggested to improve myocardial ischaemia and protect cardiac progenitor cells.46 They also exert protective effects independent of GLP-1 receptors.6 Liraglutide has been reported to induce endothelial nitric oxide synthase via AMP-activated protein kinase signalling.6 Nitric oxide production improves coronary artery flow and endothelial dysfunction. Besides, GLP-1 RAs inhibit mitochondrial oxidative damage and attenuate reactive oxygen species production.47 Liraglutide has also been shown to suppress vascular cell adhesion molecule-1 expression in the endothelium.6 It also improves arterial stiffness and LV function, while reducing NT-proBNP levels, a biomarker for LV dysfunction.48 Vasodilatory and antioxidant actions could account for some of the antiatherogenic effects in GLP-1 RAs, therefore this drug class may be preferable in diabetic patients with predominant ASCVD risk.3
However, different results were found in studies of exenatide.15,20 Intravenous exenatide in patients after coronary artery bypass grafting surgery did not offer additional cardiovascular benefits compared to parenteral insulin.20 Differences have been suggested to be related to different immunogenicity profiles and signalling pathways in exendin-4 and GLP-1 based agonists.46 Exendin-4 based agonists are postulated to be more immunogenic and cause injection site reactions, leading to higher drug discontinuation rate and diminished benefits in the EXSCEL trial.15,46 Given the conflicting evidence, CVOTs were conducted to study GLP-1 RAs with different populations and formulations. The FREEDOM-CVO and REWIND trials included injection-free GLP-1 RA with osmotic mini-pump and patients without established cardiovascular background respectively, whereas the PIONEER 6 study investigated oral semaglutide.49–51 Mortality outcomes in some of these trials are encouraging, yet the results are inconsistent.49,51
GLP-1 RAs uniquely reduced nonfatal stroke events in the LEADER and SUSTAIN-6 trials.13,52 The effects were confirmed in our latest NMA (OR 0.88; 95% CI [0.77–0.99]).17 GLP-1 RAs are reported to be neuroprotective because of the ability to cross the blood-brain barrier and actions on neuroinflammation pathways.47 Besides atherosclerosis, oxidative stress is considered to be responsible for stroke development.53 GLP-1 RAs reduce oxidative stress and reactive oxygen species production via p-AKT/endothelial nitric oxide synthase and nuclear factor-κ B p65 pathways.47
CVOTs are being conducted in order to expand the indication of GLP-1 RAs from ASCVD prevention in diabetes to a broader patient population. Results from the FIGHT trial did not support the use of liraglutide in HF patients, which is not surprising given less clear-cut HF benefits in NMA compared to SGLT2 inhibitors.17,54 The on-going EGRABIS1 (NCT02829502) and Lirabolic (NCT04057261) trials also aim to explore the effects of GLP-1 RAs on cardiometabolic markers, such as mean cerebral flow velocity, as well as BP and lipid profiles. Together with the SLIM LIVER (NCT04216589) study in non-alcoholic fatty liver disease patients, these trials will clarify the neuroprotective and cardioprotective mechanisms of GLP-1 RAs independent of glucose levels. This might provide the scientific basis for the benefits of this drug class in the prevention of vascular diseases.
DPP-4 Inhibitors
DPP-4 inhibitors did not show cardiovascular benefits in meta-analyses by other researchers and ourselves.16,17,55 A previous meta-analysis concluded significant reductions in MACE, but the inclusion of trials with shorter follow-up periods may limit the robustness of the conclusions. Elevated hospitalisation for HF incidence remains a concern, as reported in the SAVOR-TIMI 53 trial.55–57 However, in the EXAMINE and the latest CARMELINA trials, HF incidences were neutral.58,59
DPP-4 inhibitors have been reported to inhibit degradation of endogenous peptides other than GLP-1, including stromal-cell-derived factor (SDF) 1, neuropeptide Y and substance P (Figure 2).60 Potentiation of these peptides results in sympathetic activation via cyclic AMP signalling and beta-receptor activation, which may contribute to HF development and cardiac fibrosis. Elevated SDF-1 and substance P levels are also suggested to induce apoptosis of the myocardium.60 In the SAVOR-TIMI 53 trial, attenuated HF elevation was detected when beta-blockers were used concomitantly.58,60 This might imply that chronic sympathetic activation might underlie the inferiority of DPP-4 inhibitors. Notably, most patients enrolled in trials with neutral HF outcomes have concomitant prescriptions for RAAS inhibitors, which could ameliorate the tendency of DPP-4 inhibitors to worsen HF.58,59 Combination therapy may counteract the detrimental effects of DPP-4 inhibitors, thus masking the harmful effects. In real life, patient with diabetes are often receiving a RAAS blocker. This makes the drug class useful even in dialysis patients.8 Nevertheless, DPP-4 inhibitors should be used with caution in patients with HF or at risk of HF.
Clinical Implications
Observational studies have been conducted to provide insights into the clinical roles of the new antidiabetic drugs.61,62 A retrospective cohort study has demonstrated beneficial safety profiles over sulphonylureas, whereas O’Brien et al. reported similar improvements in cardiovascular outcomes across the three drug classes.61,62 However, it must be noted that selection bias could be prominent in observational studies, as the number of patients included in the study was significantly higher for those receiving DPP-4 inhibitors (28,898) than for SGLT2 inhibitors (5,677) or GLP-1 RAs (11,351).62 The lack of patients enrolled in specific groups also make the use of propensity score matching difficult. Nevertheless, these studies are still valuable because head-to-head randomised controlled trials are lacking. Population-wide observational studies provide a good and generalisable estimation of clinical effects with modest confidence, thus defining the clinical roles of each antidiabetic drug class more clearly.
Choosing an antidiabetic drug class with proven cardiovascular and mortality benefits is now advocated in guidelines.3,4,63 SGLT2 inhibitors and GLP-1 RAs should be recommended as second-line treatment options after metformin, echoing recommendations by the ADA and the American College of Cardiology.3,63 The choice between SGLT2 inhibitors and GLP-1 RAs should be tailor-made according to patient characteristics. SGLT2 inhibitors offered more overall mortality benefits. They should be considered superior in most patients, including those with HF and CKD.3 However, ischaemia prevention is superior for GLP-1 RAs, thus making them preferable in patients with predominant ASCVD risk.3 Differences in routes of administration and adverse effect profiles may also play a role in prescription decisions. Most GLP-1 RAs are available as subcutaneous injections, which are inconvenient. Oral semaglutide might make this class attractive to use, but it is expensive. On-going trials, including head-to-head trials, are required to address comparative safety and efficacy, as well as possible intraclass differences.
Conclusion
Current evidence confirmed the cardiovascular safety of the three new antidiabetic drug classes, but it is important to appreciate the differences among them. SGLT2 inhibitors show superiority in mortality and cardiovascular events, mainly driven by hospitalisation for HF, and renal events. GLP-1 RAs can reduce nonfatal stroke and MACE, yet inconsistent evidence suggests possible intraclass differences. Both drug classes should now be considered as the preferred second-line treatment in T2D patients after metformin according to patient characteristics. In terms of cardiovascular and renal outcomes, DPP-4 inhibitors have not demonstrated benefits in comparison with placebo and have been proven to be inferior to GLP-1 RAs and SGLT2 inhibitors. In this new era, antidiabetic drugs no longer just control blood glucose, but should also address the cardiovascular risks of T2D patients.