Heart failure (HF) with preserved ejection fraction (HFpEF) and AF are two of the most common cardiovascular conditions encountered in daily practice, and are leading causes of hospitalisation and adverse patient outcomes.1,2 Both conditions have increasing prevalence and pose a growing burden on global healthcare systems. They share common risk factors, such as hypertension and obesity, that are themselves increasing in prevalence. Patients with HFpEF or AF are often multimorbid, with advanced age, ischaemic heart disease, diabetes and other non-cardiovascular conditions. HFpEF and AF frequently coexist, and each predisposes to the other. Patients with an already high risk of adverse events (including death) have an even worse prognosis when HFpEF and AF combine.3
In this review we propose various targets to break the cycle between HFpEF and AF, learning lessons from epidemiology, pathophysiology and associated comorbidities to improve diagnosis, treatment and patient well-being. In the rapidly developing fields of HFpEF and AF, we outline where joined-up thinking can help both elements independently, as well as create a new treatment paradigm for patients with both HFpEF and AF.
Epidemiology of HFpEF and AF
Studies of incident HF in community-based cohorts suggest that HFpEF accounts for between 37% and 53% of HF cases overall, with a higher observed incidence in older participants.4–6 These figures are likely an underestimate of the burden of HFpEF due to the challenges in diagnosing HFpEF, particularly in primary care settings. Hospitalisation due to HF is responsible for a significant proportion of the cost burden of cardiovascular diseases. Although HF with reduced ejection fraction (HFrEF) costs more than HFpEF on an individual basis, the increase in acute admissions is being driven more by HFpEF.7,8
With regard to AF, the projected prevalence is rising rapidly, fuelled by better identification, an ageing population and improved survival from acute coronary syndromes and HF, which, in later life, can increase the risk of AF.9 With AF associated with high rates of stroke and thromboembolism, as well as evidence of a clear link with cognitive decline and vascular dementia, the increase in AF across all communities will have a profound public health impact.10
The reported prevalence of AF in HFpEF (and vice versa) varies widely, likely due to differences in HFpEF definition, HF severity and, in particular, study selection criteria. For example, research involving AF interventions tends to have lower rates of HFpEF compared with HF interventions reporting AF.11–13 In the long-term HF registry of the European Society of Cardiology, the prevalence of AF was 39% among HFpEF patients.14 Again, this is likely an underestimate because it does not consider the temporal relationship between these conditions.15 In Framingham participants with new-onset HF (1980–2012), the overall rate of AF in HFpEF (considering previous, concurrent and future AF) was 62%; this was significantly higher than the 55% of patients with AF in the context of HFrEF.16 Based on these figures, the prevailing notion that AF is common in HFpEF is wrong; in fact, over time, having concomitant HFpEF and AF is actually the norm. This has major implications on the ability of cardiologists and general physicians to improve patient outcomes, as discussed below.
Mechanisms Underlying Both Heart Failure With Preserved Ejection Fraction and AF
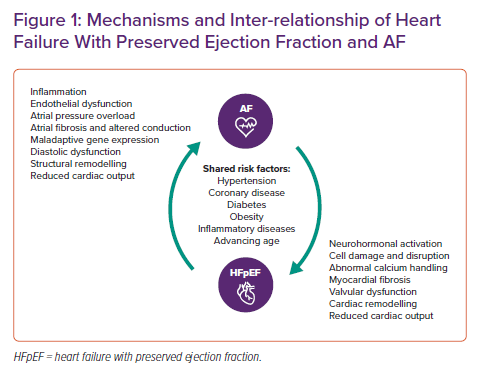
There are multilevel links between HF and AF, contributing both simple and complex mechanisms that lead to concurrence in individual patients. The inter-relationship between HFpEF and AF is outlined in Figure 1 and includes cellular, biohumoral, structural and haemodynamic changes from HFpEF and AF that cause the progression of each condition and increase the likelihood of the other developing. Much has been made of this cyclical relationship in the literature, but probably more important to shared pathophysiology and reciprocal causation is the connection to a set of similar comorbidities. Early ageing, hypertension, coronary artery disease, diabetes, obesity and a range of other comorbidities are all antecedents of both HFpEF and AF, many with inflammation as an underlying trigger. The sequence that links inflammation to HFpEF, AF and both diseases combined includes endothelial dysfunction and oxidative stress, culminating in end-organ manifestations, such as diastolic dysfunction.17 In addition, multimorbidity is increasing in patients with HF, as demonstrated by a longitudinal study in which 87% had three or more comorbidities in 2012-14, compared to 68% a decade previously.18 The interactions of these comorbidities will place additional burden on the mechanisms portending to HFpEF and AF.
With regard to more specific cardiac interactions, left atrial structural and functional remodelling is a clear mechanism through which HFpEF leads to AF. Left atrial enlargement and pressure change is commonly associated with a proarrhythmic substrate due to atrial fibrosis, which promotes further electrical remodelling, decreases the atrial effective refractory period and enhances the risk and burden of AF.1,19 Subsequent upregulation of the adrenergic and renin–angiotensin–aldosterone systems can accentuate atrial fibrosis, and changes to atrial and ventricular natriuretic peptide release and other neurohormonal activation and haemodynamic changes can trigger the development of ventricular myocardial fibrosis. This and the resultant structural changes (often made worse by valve dysfunction) further worsen HFpEF status and set up a continuum of deteriorating cardiac output. Added to this, persistent tachycardia from uncontrolled AF can contribute to both an atrial and ventricular cardiomyopathy.20
Prognostic Implications
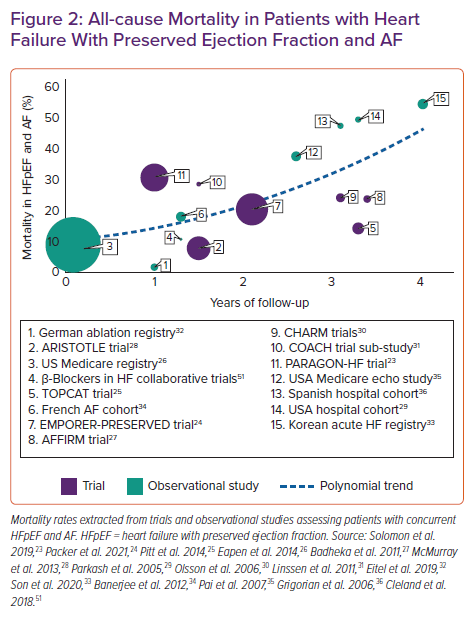
The poor outlook for patients with either HFpEF or persistent forms of AF is further worsened when these conditions combine, augmented by the impact of interacting comorbidities and varying due to the heterogeneity of HFpEF.16,21,22 Adverse event rates are generally increased, most notably death. Incident AF can double mortality risk in patients with HFpEF, independent of underlying risk factors.15 Extrapolating from the published sources described in Figure 2, average absolute mortality rates are approximately 20% at 2 years in patients with combined HFpEF and AF, increasing to around 45% at 4 years. In a meta-analysis of 45,100 patients, the increase in mortality was higher when HFrEF was combined with AF (relative risk 1.24 versus HFpEF-AF; 95% CI [1.12–1.36]; p<0.001).37 However, there was no significant difference between HFrEF-AF and HFpEF-AF in the rates of hospitalisation due to HF, or incident stroke. Patients with HF and AF have poor quality of life, substantially worse across most domains than other long-standing illnesses, with a negative trajectory over time and more patients deteriorating than improving.38,39
Diagnostic Challenges for Heart Failure With Preserved Ejection Fraction and AF
Different trials, observational studies and registries have used varying definitions of HFpEF, including various cut-off points for left ventricular ejection fraction (LVEF). However, the diagnosis of HFpEF requires more than just ‘normal’ LVEF. Current guidelines from the European Society of Cardiology define HFpEF as patients with a clinical syndrome of HF (with characteristic symptoms and signs), a consistent rise in natriuretic peptides and some objective evidence of diastolic impairment.40 Each of these aspects poses particular difficulties in the context of concomitant AF. Symptoms such as dyspnoea and lethargy are common to both HFpEF and AF, and natriuretic peptides are elevated in patients with AF regardless of HF status, especially in those with persistent forms of AF. In a recent healthcare-embedded clinical trial of patients with permanent AF and dyspnoea (New York Heart Association [NYHA] class II or above), the median N-terminal pro B-type natriuretic peptide (NT-proBNP) concentration was 1057 pg/ml (interquartile range 744–1522 pg/ml), a magnitude higher than the usual cut-off point used to exclude HF.41
Documenting diastolic dysfunction using cardiac imaging is also challenging when AF is present, and there is limited information about what measurement and what value should be used in these patients.42 The current guideline-suggested practice of averaging multiple sequential beats in AF to obtain a reasonable mean is not based on scientific principle. Variation between beats for the measurement of E/e′ filling pressures is over 40% in AF, meaning that reproducibility is so low that we should question the value of such measurements.43 In contrast, the index beat approach selects appropriate cardiac cycles for measurement, thereby addressing beat-to-beat variation in AF. In a blinded study, this physiology-based approach was more reliable (coefficient of variation reduced to 25% for E/e′) and more efficient in terms of echocardiographer time.43
The diagnosis of AF benefits from many new forms of rhythm monitoring, including consumer electronic devices such as smartwatches. However, the validation of these devices remains unclear, and the AF they describe may not be associated with the same degree of impact on stroke and thromboembolism.44,45 Incident cardiac and cerebral event rates may also be dependent on the ‘severity’ of HFpEF and the degree of underlying systolic impairment in AF, even if above an LVEF of 50%.46 The value of additional physiological assessment remains unclear (e.g. exercise echocardiography, right heart catheterisation or detailed assessment of atrial function), although these tests can be valuable in particular cases where the HFpEF diagnosis is uncertain. Atrial-specific biomarkers and novel molecular imaging technologies may provide more tangible benefit in the future.47
Distinguishing the relative impact of HFpEF and AF on patient symptoms when both conditions are present is also very challenging. This can impair the ability of clinicians to use focused treatments to improve patient quality of life, especially in the presence of comorbidities.38 In some cases, the response to therapy should be evaluated; for example, how well diuretics relieve the dyspnoea and congestion of HFpEF, and perhaps subsequently decrease sympathetic drive and heart rate.1 Conversely (and where feasible), it may be useful to perform electrical or pharmacological cardioversion to assess the impact of AF rhythm on current HF symptoms, albeit that sinus rhythm may be short lasting. These approaches can help identify patients with HFpEF and AF who may benefit from additional intervention, including advanced HF therapy and AF ablation. Assessment of health-related quality of life can be performed using various tools, including generic assessments such as the EQ-5D and 36-Item Short Form Health Survey (SF-36) or disease-specific questionnaires such as Atrial Fibrillation Effect on QualiTy-of-Life.41 For HF, lower scores with the Kansas City Cardiomyopathy Questionnaire have been associated with higher all-cause death and HF hospitalisation in both HFrEF and HFpEF.48
Treatment Paradigm in Patients with Heart Failure With Preserved Ejection Fraction and AF
Patients with both HFpEF and AF require a different approach to management that encompasses the key elements of management for each condition, but also respects the interconnected nature of both and their combined effect on therapeutic efficacy. A patient-centred, shared management approach is essential,49 focused on the key outcomes of importance to that individual patient, rather than esoteric outcomes taken from clinical research studies. As patients with concomitant HFpEF and AF are often older, more comorbid and already dealing with polypharmacy, it may be more relevant to focus on aspects that improve quality of life. In contrast, some patients will give a clear steer about their desire for prognostic improvement. Whichever approach is prioritised, feedback about progress can inform future clinical decisions, and tools such as quality of life questionnaires can be helpful in evaluating effectiveness and residual impairment.38
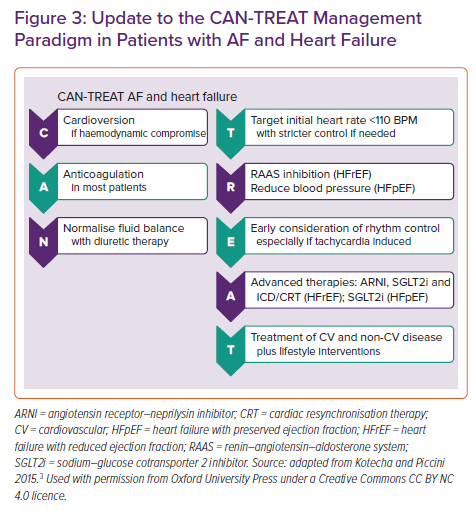
Key steps in the management of patients with HF and AF are presented in Figure 3 (the CAN-TREAT algorithm).3,50 Most treatment steps are similar regardless of the LVEF of the individual patient, reflecting the need to ensure haemodynamic stability first and foremost, more widespread use of anticoagulation to prevent thromboembolism and achieving euvolaemia. More specific approaches for rate control, heart failure therapy and rhythm control then diverge for patients with HFpEF compared with HFrEF. The intermediate group, also known as HF with mildly reduced ejection fraction, should be treated as though they have HFrEF due to the consistent evidence that they benefit from HFrEF treatments.51,52 An often-neglected component of the care of patients with HF and AF is to carefully and systematically address comorbidities; not just hypertension and myocardial ischaemia, but also non-cardiovascular diseases. This requires an integrated approach to achieve the best outcomes, not only between HF and AF clinical teams, but also the spectrum of healthcare professionals. Finally, to achieve the best outcomes, the conventional ‘sequential’ management approach should be discontinued. Relevant components of the treatment algorithm can be started in parallel; for example, starting new therapies without waiting to fully uptitrate prior drugs. Such an approach has already been advocated for HFrEF.53
Prevention of Thromboembolism
Anticoagulation is one of the only therapeutic approaches in AF with clear and proven ability to improve prognosis.54 Although there are no specific trials in HFpEF, post hoc analysis of the four major trials of direct oral anticoagulants (DOACs) showed similar efficacy in those with HF. Compared with warfarin, DOACs reduced the risk of stroke and systemic embolisms in HF patients by 14%, with a 24% lower risk of major bleeding.55 Therefore, except in the case of patients with severe mitral stenosis, mechanical valve prosthesis or end-stage renal dysfunction, DOACs are the first-line approach for the prevention of thromboembolism in HFpEF and AF. The place of percutaneous left atrial appendage closure is unclear due to the lack of trials against DOAC therapy. Current guidelines indicate the use of percutaneous left atrial appendage closure only in cases with an absolute contraindication for DOAC therapy (e.g. intracranial bleed without a reversible cause).56 Where available, thoracoscopic left atrial appendage clipping is also an option in this patient group.57
Therapies Targeting the Heart Failure With Preserved Ejection Fraction Component
Attention to fluid balance and diuretic dose is crucial, and euvolaemia should be achieved as a priority to avoid driving tachycardia and neurohormonal activation. The use of treatments with proven benefit in HFrEF have shown disappointing results in clinical trials for patients with HFpEF. However, few of these studies have had sufficient numbers of patients with concomitant AF to be certain about either benefit or harm (SupplementaryTable 1). This applies to angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers and mineralocorticoid receptor antagonists.51,58,60–63 Specifically, the IMPRESS-AF trial randomised 250 patients with AF and HFpEF (LVEF ≥55%) to spironolactone or placebo and disappointingly identified no benefit with regard to peak oxygen consumption on cardiopulmonary exercise testing at 2 years or secondary endpoints such as 6-min walk distance, the E/e′ ratio or quality of life.64 All participants had controlled blood pressure, so there remains potential for these therapies in the context of hypertension, as discussed later.59,60
With regard to newer HF treatments, sacubitril/valsartan did not improve the composite of cardiovascular death and HF hospitalisation in patients with LVEF ≥45% (rate ratio 0.87; 95% CI [0.75–1.01]; p=0.06), and the effect in those with AF was also not significant (rate ratio 0.83; 95% CI [0.69–1.00]).23 Interaction analysis suggested those with a history of AF experienced a 13% reduction in primary event rate with sacubitril/valsartan compared with valsartan, but this ranged from a 37% greater benefit to a 21% weaker effect.23 Results from the EMPEROR-PRESERVED trial provide the first suggestion of hope for the treatment of HFpEF.65 Empagliflozin reduced the risk of cardiovascular death or HF hospitalisation (HR 0.79; 95% CI [0.69–0.90]; p<0.001) and the effects were maintained in those with AF at baseline (HR 0.78; 95% [0.66–0.93]).24 Interpretation of that trial should consider that the LVEF criterion was ≥40%. There appeared to be a ‘dose’ relationship with LVEF (more effect at lower LVEF), although the effect of empagliflozin remained significant in the subgroup of patients with LVEF 50–60% (HR 0.80; 95% CI [0.64–0.99]). These results suggest that sodium–glucose cotransporter 2 (SGLT2) inhibitors should become standard of care in both HFrEF and HFpEF, with an important role to play in patients with concurrent AF.
Therapies Targeting the AF Component
Heart rate control is often required in the management of patients with HFpEF and AF; however, care should be taken to avoid inducing bradycardia because chronotropic incompetence is common in elderly patients.66 The optimal heart rate range is not known (and likely varies for individual patients), but trial evidence suggests that strict control is not beneficial and may even increase the need for hospitalisation.67 The close linear association between heart rate and mortality seen in those with HF and sinus rhythm was not demonstrated in patients with concomitant AF.68 The choice of rate control agent is between β-blockers (most commonly used), digoxin or calcium channel blockers, such as diltiazem or verapamil. Amiodarone should not be used as a rate control agent due to its non-cardiac side effects. Comparing β-blockers versus low-dose digoxin, the RATE-AF trial randomised 160 patients with permanent AF and symptoms of HF (NYHA class II or above; 81% with LVEF ≥50%).41 The trial found no difference in the physical component of quality of life, but there were significant benefits from digoxin on AF symptoms (two-class improvement in 53% of patients versus in 9% of patients with β-blockers; p<0.001), NYHA class (mean 2.4, decreasing to 1.5 at 12 months, versus 2.0 with β-blockers; p<0.001), and NT-proBNP (960 pg/ml at 12 months versus 1250 pg/ml with β-blockers; p=0.005).41 There were also substantially fewer adverse events with low-dose digoxin (25% of patients with at least one event versus 64% with β-blockers; p<0.001). Calcium channel blockers are another option for rate control in those with normal LVEF, but their benefit on exercise capacity and NT-proBNP compared with β-blockers has only been established in a small cross-over trial of patients without HF.69 Ablation of the atrioventricular node is an option when other attempts to control heart rate fail, but leads to pacemaker dependency.
For those with ongoing symptoms despite good heart rate control, rhythm control should be considered, balancing the risk of rhythm control approaches (anti-arrhythmic drugs, such as amiodarone and dronedarone, and catheter ablation) against the potential for long-term benefit. This balance is often challenging in patients with established HFpEF and AF because, due to multimorbidity, there may be a lower chance for maintenance of sinus rhythm. Clinical trials are lacking in this patient group, and observational data are not helpful due to the considerable selection and performance biases.70 Consideration of early rhythm control, for example within the first year of AF, merits separate attention. In the EAST trial, which randomised patients to early or conventional rhythm control, the subgroup of 798 patients with HF had a similar reduction as non-HF patients for the composite of cardiovascular death, stroke and hospitalisation for HF or acute coronary syndrome (HR 0.74 with an early approach; 95% CI [0.56–0.97]; p=0.03).71 There were insufficient patients to be certain about benefit in those specifically with HFpEF (p=0.24) but, pending further trial evidence, an early rhythm approach should be considered, particularly where HFpEF may be a consequence of AF-related irregularity or elevated heart rate (e.g. in tachycardia-related cardiomyopathy). Data specifically on HFpEF are not available, although studies in HFrEF would suggest clinical benefit from AF ablation.
Lifestyle Interventions and Comorbidity Management
Lifestyle changes should be suggested in all patients with HFpEF and AF where relevant to that individual patient; this will enable the patient to take an active role in their management alongside medical therapy. Certain interventions have also demonstrated improvements in patient well-being, although there remains uncertainty about their impact on clinical endpoints.72 For example, in a meta-analysis of six small randomised trials (total of 276 patients with HFpEF), exercise training improved peak oxygen uptake (weighted mean difference 2.72 ml/kg/min; 95% CI [1.79–3.65 ml/kg/min]) and quality of life (weighted mean difference –3.97; 95% CI [–7.21, –0.72]).73 In a factorial trial of 100 obese patients with HFpEF, the addition of a low-calorie diet to an exercise regime resulted in further improvements to peak VO2 (1.2, 1.3 and 2.5 ml/kg/min for exercise, diet and both together, respectively).74 The effects of these approaches in patients with combined HFpEF and AF is not yet known.
Adequate control of blood pressure is often asserted as an essential component of HFpEF management, although there remains little trial evidence for an impact on clinical endpoints once HFpEF is established. The aforementioned trials of renin–angiotensin–aldosterone system antagonists (Supplementary Table 1) all demonstrate a significant reduction in blood pressure, so these are suitable as first-line agents until the place of SGLT2 inhibitors becomes clearer. With regard to AF, an individual patient-level meta-analysis comprising 22 trials showed that blood pressure-lowering treatment reduces the risk of major cardiovascular events to a similar extent in individuals with and without AF (13,266 and 175,304 participants, respectively).75 Each 5-mmHg reduction in blood pressure lowered the risk of stroke, ischaemic heart disease or HF by 9% during a 4.5-year follow-up. The target for blood pressure control in either HFpEF of AF remains the subject of debate.76
Other important comorbidities whose management should be prioritised in the context of HFpEF with AF are underlying ischaemic heart disease, obesity, iron deficiency and glycaemic control in patients with diabetes. Depression is common in HF patients, and although therapies can improve quality of life, the impact on clinical outcomes remains uncertain.77 Non-cardiovascular comorbidities, such as pulmonary disease, have a worse impact on mortality in HFpEF than HFrEF, whereas gout and cancer have a similar effect in both HF phenotypes.78 For AF, targeted therapy of underlying conditions in a randomised trial led to significant improvements in the maintenance of sinus rhythm.79 In HFpEF and AF, mortality and morbidity are frequently from non-cardiovascular causes, and so the management of patients is incomplete without the systematic, individualised assessment and treatment of comorbidities.80
Conclusion
HFpEF and AF are increasingly prevalent and, when combined, lead to a substantial increase in mortality and poor patient quality of life. Diagnosis is challenging and conventional therapy is often unable to improve clinical endpoints. A paradigm shift is needed in clinical management that considers the joint effects of both conditions in order to adequately treat patients. This approach should be personalised, use non-sequential treatment prescription targeted to both HFpEF and AF components and integrate attention on underlying comorbidities to prevent progression.