Ideally biomarkers provide the clinician with assistance in one or more of: (i) diagnosis, (ii) prognosis, (iii) choice and titration of therapy, (iv) monitoring progression of disease and (v) assessing response to treatment. The best-established biomarkers in acute decompensated heart failure (ADHF) are the B-type natriuretic peptides (brain natriuretic peptide [BNP] and N-terminal of the prohormone brain natriuretic peptide [NT-proBNP]). Their application in the diagnosis of ADHF and in risk stratification in both acute and chronic HF (AHF/CHF) is now endorsed by all major international guidelines for the diagnosis and management of HF.1,2 This brief review will summarise the main evidence underpinning this status and also outline the shortcomings of B-type cardiac peptides as diagnostic aids in ADHF. Acute and chronic kidney dysfunction and injury frequently complicate, and confer a worse prognosis in, ADHF. The impact of kidney function upon the test performance of BNP/NT-proBNP in ADHF and the currently unmet need for reliable markers of acute kidney injury (AKI) in the context of ADHF are briefly outlined.
Cardiac
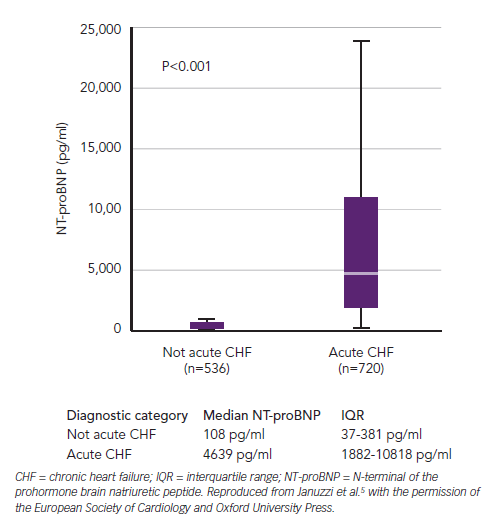
The landmark Breathing Not Properly, International Collaborative of NT-proBNP Study (ICON) and Biomarkers in Acute Heart Failure (BACH) trials followed small proof-of-principle reports confirming the diagnostic performance of B-type cardiac natriuretic peptides in AHF (see Figure 1), and defining cut-points for BNP and NT-proBNP as diagnostic aids for identifying ADHF in the emergency department (ED).3–6 Robust ‘rule-out’ values with both sensitivity and negative predictive values over 90 % for the diagnosis of ADHF in those presenting acutely to the ED with the primary complaint of breathlessness are 100 pg/ml and 300 pg/ml for BNP and NT-proBNP, respectively (see Figure 1 and Table 1).4,5 This overall performance is reliable but defined subgroups require additional interpretation and elevation of B-type natriuretic peptides is not specific to ADHF. Box 1 offers a differential diagnosis (atrial fibrillation [AF], pulmonary embolism, etc.), which should be considered when elevated test results become available. As is the case for many medical diagnostic challenges, the BNP/NT-proBNP levels should not be considered in isolation but as part of the usual armamentarium of history, examination and corroboration by other tests.
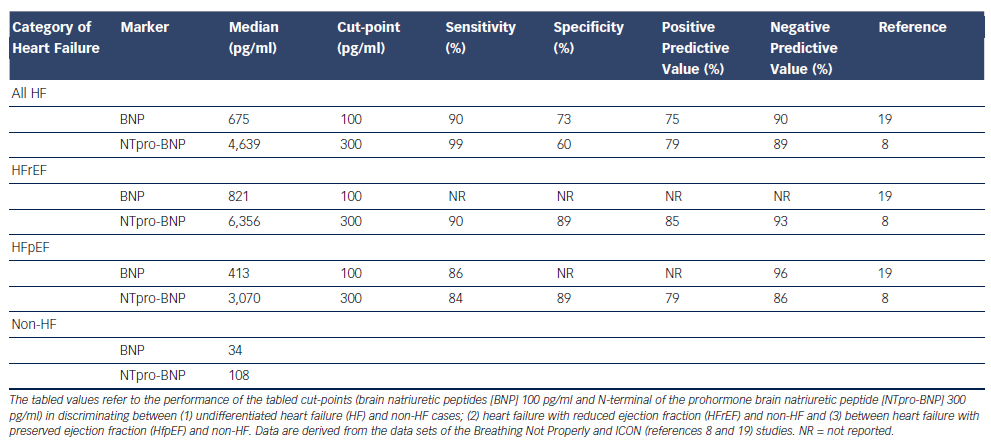
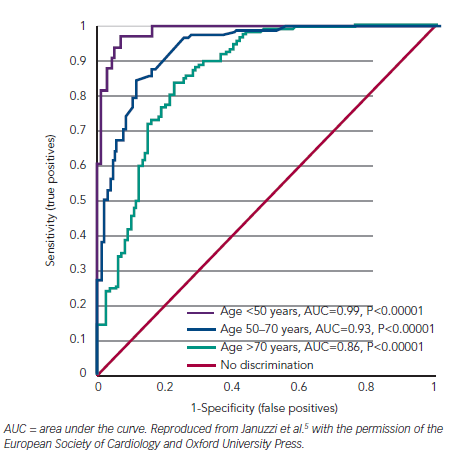
Consideration of other confounding influences upon all cardiac natriuretic peptides (i.e. atrial natriuretic peptide [ANP], BNP and C-type natriuretic peptide [CNP]; collectively NP) plasma concentrations is necessary for a fully informed interpretation of plasma B-type natriuretic peptide results. First, age is an independent determinant of plasma concentrations of all NP. This does not invalidate the rule-in values given above but, for enhanced specificity, once the rule-out value is exceeded, consideration of age-adjusted values do enhance accuracy (see Table 1; Figure 2). In people 50 years of age or less, an NT-proBNP level of 450 pg/ml or more in the presence of acute breathlessness is almost a perfect test for ADHF (area under the curve [AUC] 0.99). Of course, most of our HF patients are older and the AUC falls to 0.93 at 50 to 75 years (when the optimal test level is 900 pg/ml) and to 0.86 for those over 75 years (1,800 pg/ml). These age-adjusted values are well-validated for NT-proBNP, but have not been formally addressed for BNP.5
Second, whether or not left ventricular ejection fraction (LVEF) is reduced (HFrEF) or preserved (HFpEF) in HF influences plasma B-type natriuretic peptides, with levels in HFpEF being approximately half those seen in HFrEF (see Table 1) in both AHF and CHF.7–9 This reflects the integrated influence of ventricular internal dimensions, wall thickness and intra-ventricular pressures (embodied in the law of Laplace) upon cardiomyocyte stretch, the primary driver of NP synthesis and release. However, because the ‘signal to noise’ ratio in BNP testing is so high in AHF, which is characterised by quite extreme elevations in BNP/NT-proBNP, the performance of the test in HFPEF is only slightly reduced (see Box 1). In contrast in the settings of incipient or treated HF, NP values often fall into the sub-diagnostic range – particularly so in HFPEF.10,11 This emphasizes the need to apply the recommended cut-point values for AHF in the appropriate setting; that is, in patients with new onset of distressing breathlessness in whom AHF is plausible.
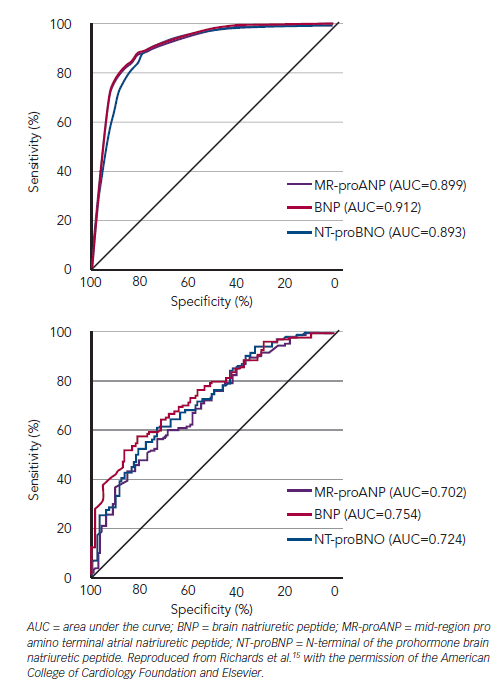
Third, AF, present in approximately 30 % of populations with newly presenting AHF, elevates plasma NP in presence or absence of HF.12–14 The discriminative power of BNP/NT-proBNP for ADHF in newly dyspnoeic patients is clearly reduced with AUCs falling from about 0.9 for those in sinus rhythm to ~0.7 in AF (see Figure 3).15 Sensitivity is preserved (because overall NP levels are raised by AF), but specificity and accuracy are markedly reduced and cannot be improved by merely altering the cut-point chosen although some suggest a higher decision level of 200 pg/ml for BNP in AF. In fact between 65 and 85 % of breathless patients presenting acutely with complicating AF with B peptide levels above 100 pg/ml and 300 pg/ml for BNP and NT-proBNP, respectively, will indeed have HF and it is rational to regard such patients as having ADHF until proved otherwise.12–15
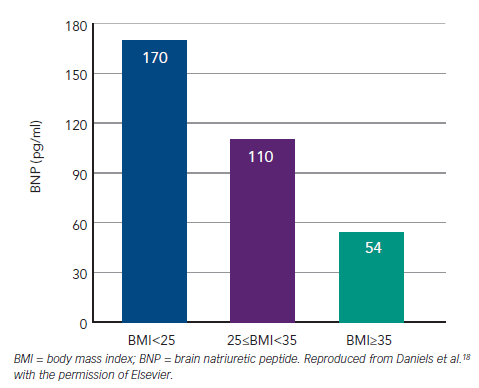
Fourth, body mass index (BMI) is inversely related to plasma NP concentrations in both health and HF. The underlying mechanisms are not understood.16–18 Unlike renal impairment or AF, obesity leaves the overall discrimination of BNP for AHF largely preserved with AUCs only moving from 0.9 to 0.88 between BMI <25 and >35 kg/m2. The effect is sufficiently strong to reduce the sensitivity of BNP at 100 pg/ml and analysis from the trial led to a recommendation to reduce the cut-point to 50 pg/ml where obesity (i.e. BMI >30 kg/m2 ) is present (see Figure 4).18 Test performance of NT-proBNP appears less affected by obesity.17
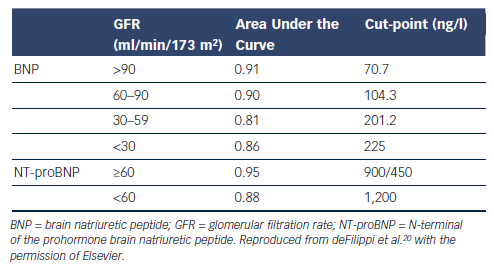
Fifth, plasma creatinine and estimated glomerular filtration rate (eGFR) are inversely related to plasma NP levels.4,5,19 This is a sufficiently strong effect to lead to the recommendation that the cut-point for BNP should be increased to 200 pg/ml when eGFR falls below 60 ml/min/1.73m2.20 No specific corresponding change in cutpoint is generally applied to NT-proBNP values and the performance of NT-proBNP is less affected (see Table 2).20 For both markers, specificity and accuracy are somewhat reduced regardless of any alternative choice of decision thresholds.
The advent of combined angiotensin receptor blocker/neprilysin inhibitor drugs (ARNIs) is a recent addition to potential confounders. These agents impede metabolism of the bioactive carboxy terminal forms of ANP, BNP and CNP. BNP levels are increased slightly with early dosing. Effects upon the more avid substrates for neprilysin, ANP and CNP have not been documented. With beneficial effects upon intra-cardiac filling pressures and possible improved ventricular remodelling consequent to ARNI therapy, it is likely endogenous secretion of NPs will fall. It seems likely the signal provided by BNP will not be overly confounded. However, in our current state of knowledge this is an instance where preferential use of NT-proBNP rather than BNP is warranted as neprilysin inhibition will not directly affect clearance of the amino terminal portion of any of the NPs.
Many new candidate markers for HF are under scrutiny. Topical ‘newcomers’ include mid-region pro-adrenomedullin (MR-proADM), soluble suppression of tumorigenicity 2 (ST-2), growth differentiation factor 15 (GDF 15) and Galectin 3.6,21–23 Several have been endorsed in international guidelines as offering independent prognostic information in acute and CHF and they offer more fine-grained risk stratification when combined with BNP/NT-proBNP.2,21–23 MR-proADM in particular is superior to BNP/NT-proBNP for prediction of mortality in the first 30 days after onset of ADHF.6,24 At the time of writing there is no new marker that offers diagnostic performance superior to BNP/NT-proBNP.
Kidney
ADHF is associated with a high incidence (~25 %) of AKI, often superimposed on pre-existing chronic renal impairment. In unselected series half of patients with CHF have an estimated eGFR below 60 ml/min/1.73m2.25,26 AKI usually manifests in the first few days of admission and is most commonly regarded as type 1 cardiorenal syndrome defined as acute impairment of renal function secondary to acute cardiac insufficiency. Both background renal dysfunction and any acute decrement portend a worse prognosis in ADHF.25–28 Since 2000, reports from several large series consistently indicate that AKI, defined as an increment in plasma creatinine of over 0.3 mg/dl (i.e. 26.4 μmol/l), is associated with a near doubling of mortality at 1 year and increments in creatinine as little as 0.1 mg/ dl (9 μmol/l) are associated with worse prognosis.28
Although efforts to date to target AKI in ADHF with specific pharmacotherapies have been disappointing,29 awareness of developing kidney injury in ADHF can trigger supportive measures (avoidance of nephrotoxic drugs and contrast media, avoidance of hypotension, careful titration of any measures influencing volume and pressure homeostasis) and resulting in improved outcomes.30 Unfortunately, dependence on serial measurement of plasma creatinine to monitor renal status delays awareness of AKI by 24–72 hours. A major cause of failed intervention trials in AKI is delayed intervention resulting from the delay inherent in the use of creatinine as the diagnostic surrogate of reduced GFR, and the misconception that loss of filtration function is an early rather than a late sign of renal injury.31 Further, many patients with a near-normal serum creatinine level on admission with ADHF have evolving AKI, not apparent due to absence of information on pre-admission baseline values. In addition, whether AKI in HF from hypoperfusion differs from injury resulting from renal venous congestion is unclear.32 Hence there is an important unmet need for early markers of AKI in AHF in order to allow timely introduction of supportive measures and to target later experimental therapies for AKI in ADHF.
Many recently identified biomarkers of renal cellular damage, such as kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL) and interleukin-18 (IL-18), facilitate earlier detection of AKI following ischaemic and nephrotoxic kidney injury.31,33–35 None have entered routine clinical practice. It is unlikely a single biomarker could identify AKI due to aetiological diversity and clinical uncertainty regarding time of onset of renal insult.11
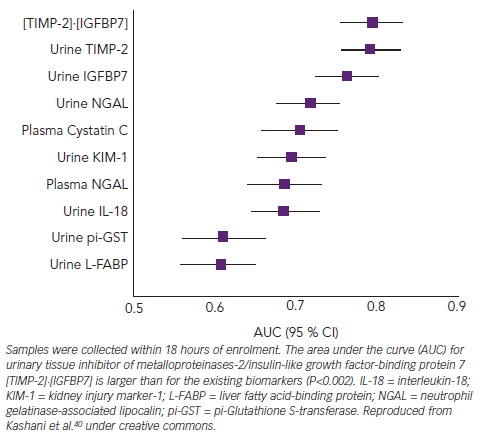
NGAL has claimed particular attention in recent years, but published reports consistently indicate the AUC for detection of AKI in ADHF using urine or serum NGAL is ~0.7.36–39 In order to offer meaningful clinical advantage, markers generally require AUCs in the order of 0.75 or more. In this regard exciting recent data from a trial in patients with heterogenous forms of serious acute disease indicate that the cell cycle markers insulin growth factor binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases type 2 (TIMP-2) perform at this level with AUC for detection of AKI of ~0.8 (see Figure 5).40 These new markers have not been tested specifically in an ADHF population.
Conclusions
BNP and NT-proBNP offer important diagnostic and prognostic information in AHF. Consideration of common background factors including age, preservation or reduction of LVEF, age, obesity and renal function will occasionally be required to help clinicians interpret B peptide concentrations in breathless patients. However, in ADHF the typical acute elevation of plasma B peptide is so profound that good discrimination is maintained in the vast majority of cases. In contrast to the relatively mature state of markers for ADHF, we are currently bereft of reliable high-performing markers of AKI with NGAL, KIM1 and other candidates so far failing to meet the performance required to aid clinical practice. IGFBP7 and TIMP-2 have shown promise as markers of AKI in a heterogenous intensive care population but are yet to be evaluated fully in ADHF.