Dear Editor,
As of 31 March 2020, the Centers for Disease Control has reported a total of 163,593 confirmed coronavirus disease 2019 (COVID-19) cases and 2,860 COVID-19-related deaths in the US. According to several public health predictive models, these numbers are expected to continue to rise in the upcoming weeks, leading to a nationwide shortage of hospital beds and especially intensive care unit (ICU) beds. Owing to its predominantly respiratory manifestations, including acute respiratory distress syndrome (ARDS), one of the treatment modalities that is expected to run short is mechanical ventilators.
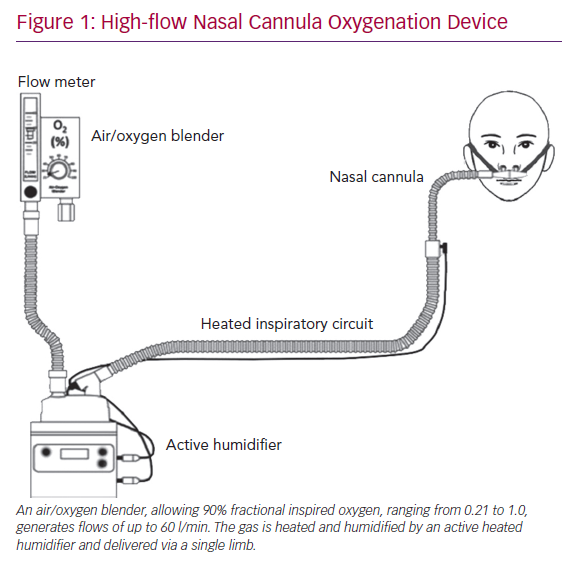
A case series of 138 COVID-19 patients from Wuhan, China showed that a total of 36 (26%) patients required ICU level care, of whom 22 (61%) developed ARDS and 17 (47.2%) required invasive mechanical ventilation.1 Other retrospective analyses have reported similarly that 20–31% of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients develop ARDS and require ICU care.2–4 Therefore, it is critical that we explore the utility and safety of other forms of respiratory support devices, including high-flow nasal cannula oxygenation (HFNCO) in the treatment of acute respiratory failure. In the above mentioned case series from China, 4 (11%) of the patients admitted to the ICU were successfully treated with HFNCO (Figure 1).1 Similarly, in other case series of 191 COVID-19 patients, 41 (21%) were treated with HFNCO (33 in ICU and 8 in non-ICU).4
We present a case of a SARS-CoV-2-positive patient with acute respiratory failure who was successfully treated with HFNCO. We also discuss the mechanisms of action, clinical effects, and available literature on the efficacy and safety of HFNCO, including the risk of aerosolising SARS-CoV-2 particles.
Case Presentation
A 51-year-old man presented to the emergency department (ED) with a 1-week history of worsening dyspnoea, fevers and non-productive cough in light of negative influenza testing at his primary care physician’s office. The patient had no travel history, but reported contact with international clients through his work.
His vital signs at the time of presentation were oral temperature of 99.7°C, heart rate of 105 BPM, respiratory rate of 35, blood pressure of 113/99 mmHg and oxygen saturation of 80% of room air. His physical examination was significant for respiratory distress, with use of accessory muscles and crackles in bilateral lung bases upon auscultation.
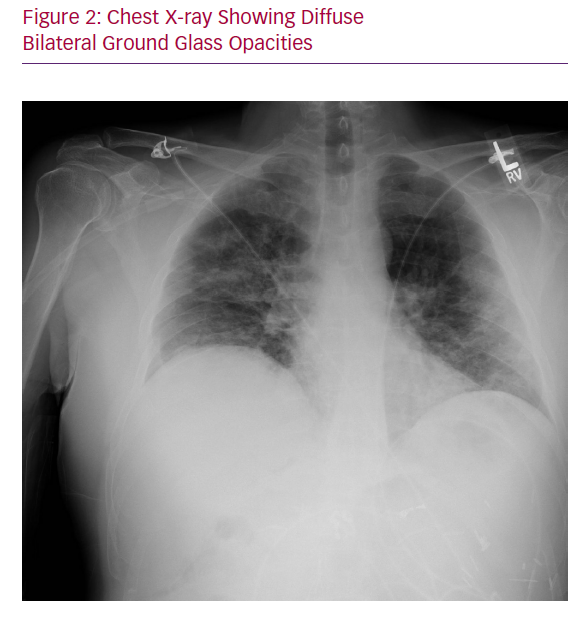
Chest X-ray showed bilateral multifocal hazy interstitial opacities (Figure 2). Respiratory viral panel, including influenza, was negative, but a SARS-CoV-2 polymerase chain reaction test that was sent while the patient was in the ED returned positive on day 4.
The patient was initially admitted to the progressive care unit under droplet and contact precautions pending SARS-CoV-2 test results, and ceftriaxone and azithromycin were initiated for presumed community-acquired pneumonia. On day 3, his hypoxic respiratory failure worsened, requiring high-flow nasal cannula at 40 l/min and 90% fractional inspired oxygen, (FiO2) and he was transferred to the medical ICU. The infectious diseases team was consulted and the patient commenced a 5-day course of lopinavir/ritonavir and hydroxychloroquine once the SARS-CoV-2 test was confirmed to be positive. Over the next 5 days, the patient was gradually weaned to room air. When he was haemodynamically stable, he was discharged with instructions to continue self-isolation at home for 14 additional days.
Discussion
Our report discusses a COVID-19 patient who presented with acute respiratory failure with moderate ARDS, in whom endotracheal intubation was prevented; the patient was successfully treated on HFNCO. The physiological benefits of HFNCO are improved oxygenation, decreased anatomical dead space, decreased metabolic demand of breathing, decreased production of carbon dioxide, superior comfort and improved work of breathing, positive nasopharyngeal and tracheal airway pressure and better secretion clearance.
First, the most important clinical benefit of HFNCO is that of efficient supplemental oxygen delivery. HFNCO therapy generates a flow-dependent FiO2.5 HFNCO therapy is able to maintain a high FiO2 by delivering flows higher than the spontaneous inspiratory demand, thus minimising room-air entrainment. In order to maximise the benefit of HFNCO, the flow rate must be titrated to match the patient’s inspiratory demand and severity of respiratory distress.
Second, HFNCO is also able to decrease anatomic dead space by washing CO2 out of the upper airways. Reduction in anatomic dead space then leads to improved work of breathing and lower respiratory rates. Mauri et al. demonstrated this effect in their study of hypoxemic patients with arterial partial pressure of oxygen to FiO2 ratios <300, where high-flow nasal cannula set at 40 l/min significantly reduced work of breathing and respiratory metabolic demand compared with oxygen delivered by face mask at 12 l/min.6 Therefore, patients with hypercarbia, in addition to hypoxaemia, gain benefit from HFNCO, not only through reduction in anatomic dead space but also through reduced CO2 production via lowered metabolic demand.
Third, HFNCO further reduces the work of breathing by optimally conditioning the delivered gas by warming and humidifying it to physiological conditions. This spares the body the energy cost of warming and humidifying inspired gas. Warm humid gas is also associated with better conductance and pulmonary compliance compared to dry and cooler gas. It also improves mucociliary function, thereby facilitating secretion clearance, decreasing risk of atelectasis and improving the ventilation/perfusion ratio and oxygenation.
Finally, HFNCO generates low-level positive pressure, which increases lung volumes and improves gas exchange. While alveolar recruitment results from the positive airway pressure, the magnitude of this effect is variable, and its clinical significance remains somewhat controversial. However, studies have estimated the positive pressure delivered through HFNCO to equal roughly 1 mm H2O for every 10 l of flow.7,8 In order to maximise the above-mentioned benefits of HFNCO, it is imperative to maintain the flow at the highest tolerated by the patient, usually at least 30–40 l/minute.
One potential concern that has been raised about the use of HFNCO in COVID-19 patients is that it could aerosolise the respiratory tract pathogen. Using evidence from several recently published studies, the WHO concluded that HFNCO does not create widespread dispersion of exhaled air, and therefore, should be associated with low risk of transmission of respiratory viruses.9 They do recommend wearing a standard medical face mask if a medical provider is within 2 m of the patient. However, a newer study showed that the distance of droplet dispersion from coughing increases by an average of 0.42 m with high-flow nasal cannula, and travelled further than the WHO-recommended 2-m safe exclusion zone.10 Based on this evidence, at our institution we ensure that COVID-19 patients on HFNCO are at the least in single-occupancy rooms with either negative pressure or high-efficiency particulate air filtration systems, and that all our healthcare workers caring for those patients wear full airborne personal protective equipment (i.e. N95 masks or equivalent, gown, gloves, goggles, hair covers and face shields).
Conclusion
HFNCO is an effective treatment modality for COVID-19-associated acute respiratory failure. Particularly in patients with mild to moderate ARDS and in negative pressure rooms, it could be a viable initial alternative to mechanical ventilation.