Heart failure (HF), a complex condition affecting the cardiovascular system, is the heart’s inability to pump and transport blood and oxygen throughout the body. This leads to the metabolic needs of multiple organs needing to be met.1 HF is further classified according to the left ventricular ejection fraction. The classification is as follows: maintained (>50%), mid-range (40–49%) and decreased levels (<40%).2
When the variables and risk factors that lead to HF admissions and hospitalisations are understood thoroughly, chances and opportunities arise to initiate preventative measures to improve and enhance patient outcomes and diminish the overall burden of HF.3
Data on global prevalence from 2019 showed how severe and impactful HF is globally, with an estimated 56.19 million cases and an age-standardised incidence of 711.90 per 100,000 people. Moreover, HF also contributed to 5.05 million years lived with disability globally.4
A biomarker is a piece of objective biological data that can be precisely, accurately and consistently quantified.5 The development of HF biomarker testing dates back many decades to the pioneering work of Braunwald et al., who examined patients’ serum levels using an early C-reactive protein (CRP) assay.6 Morrow and de Lemos outlined the features of clinically practical biomarkers.7 To build on this, Ibrahim and Januzzi described the requirements for clinically usable biomarkers in the context of HF, highlighting the significance of accuracy, availability, cost-effectiveness, and the capacity to offer specific disease-related information to aid in the diagnosis, risk stratification and management of HF.8
Biomarkers, which serve as measurable indicators of physiological and pathological processes, have emerged as promising tools for early detection and prognostication of HF. By reflecting underlying pathophysiological changes, biomarkers offer a non-invasive and objective approach to gauging the severity of HF, guiding treatment decisions, and predicting patient outcomes. Biomarkers have undeniably transformed the landscape of HF diagnosis and management. Among the extensively investigated biomarkers, B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) stand out as cardiac-specific indicators, demonstrating considerable promise in aiding HF diagnosis, risk stratification and prognostic assessment.9 This aligns with other studies claiming that BNPs are recognised as the gold standard among the many biomarkers studied in HF research over the years. Their integration into clinical practice has resulted in more timely interventions and improved patient outcomes. Nevertheless, the pursuit of novel and highly accurate biomarkers persists, driven by the urgent need for even earlier HF detection and enhanced prognostic capabilities. Ongoing research has unearthed various potential biomolecules, including cardiac troponins (cTn), galectin-3, soluble ST2 and growth differentiation factor 15.8,9
This literature review aims to examine the ever-evolving landscape of HF biomarkers, analysing their applications in early diagnosis, prognosis assessment and treatment response evaluation. We intend to draw attention to the crucial relevance of these markers in clinical practice and eventually improve HF treatment and patient care by thoroughly reviewing relevant data. Future research will be guided by a comprehensive summary of present study findings, maximising the potential of the role of biomarkers to enhance and improve HF outcomes and diminish the burden of this illness on healthcare systems and patients.
Methodology
Identifying the Research Question and Relevant Articles
The research question proposed in our review article is: What are biomarkers’ diagnostic, prognostic and therapeutic response roles in HF, and how do they improve clinical decision-making and patient outcomes?
The current study used the Scopus database to compile bibliographic data on the use of biomarkers in HF for diagnosis, prognosis and therapy response. The data extraction on the Scopus database was conducted on 27 June 2023. Scopus is a vast source of abstracts, articles and citations comprising various topics and subjects. The search included ‘biomarkers’ and ‘heart failure’ to determine relevant research articles in the title, abstract and keywords. Moreover, the terms ‘diagnosis,’ ‘prognosis,’ ‘novel’ and ‘treatment response’ found in the title fields of articles within the database were chosen for inclusion in the study for analysis.
Search Query
TITLE-ABS-KEY (“heart failure”) AND TITLE-ABS-KEY (“biomarker*”) AND TITLE (“diagnosis” OR “detection*” OR “prognosis” OR “severity*” OR “treatment response” OR “novel”) AND (LIMIT-TO (SRCTYPE, “j”)) AND (LIMIT-TO (PUBSTAGE, “final”)) AND (LIMIT-TO (SUBJAREA, “MEDI”)) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”)) AND (LIMIT-TO (LANGUAGE, “English”))
Study Selection
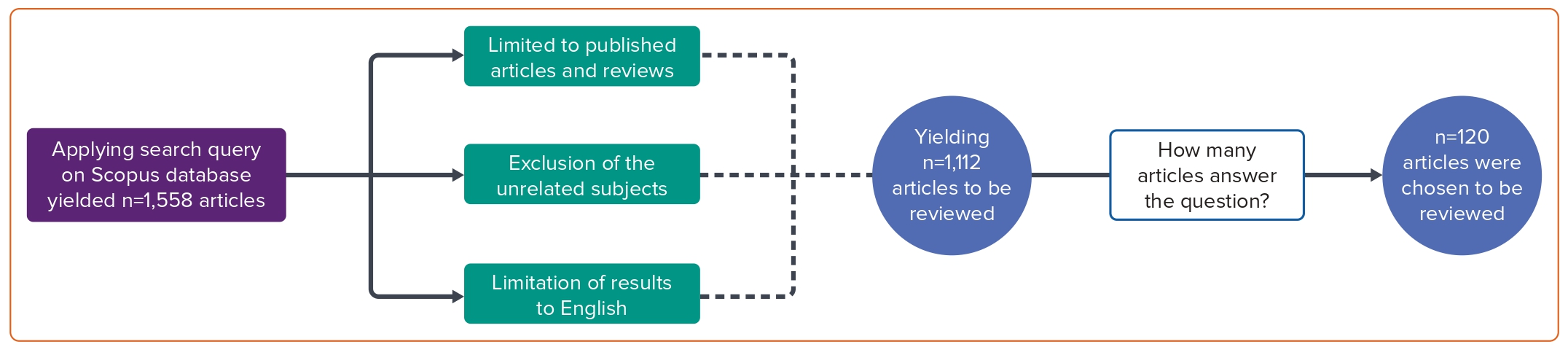
The subjects unrelated to our study, such as neuroscience, immunology, microbiology, chemistry and related fields, were excluded. The search was explicitly limited to articles on the subject of medicine. This was done to focus and limit the searches to those significant to the study’s research question. The study only included the articles if they had been published in a journal, excluding some sources, such as books. Additionally, articles were only selected if they were written and published in English. The search was not limited to a specific timeframe, as this study aimed to additionally assess the trend in using specific biomarkers over the years. With the exclusions and limitations placed, the final search query on the Scopus database revealed 1,112 studies. The authors evaluated the studies for relevancy and applicability to the research question. This resulted in the exclusion of 992 studies, leaving 120 articles to be selected for the review article (Figure 1).
Data Charting and Analysis
Microsoft Excel was used for data charting to organise the extracted data relevant to the role of biomarkers in HF for diagnosis, prognosis and treatment response assessment. Adjacent to each study title, essential study characteristics, including author names, publication year, study design and sample characteristics, were noted. Study names and associated findings were recorded on an additional sheet, allowing for a concentrated investigation of essential findings.
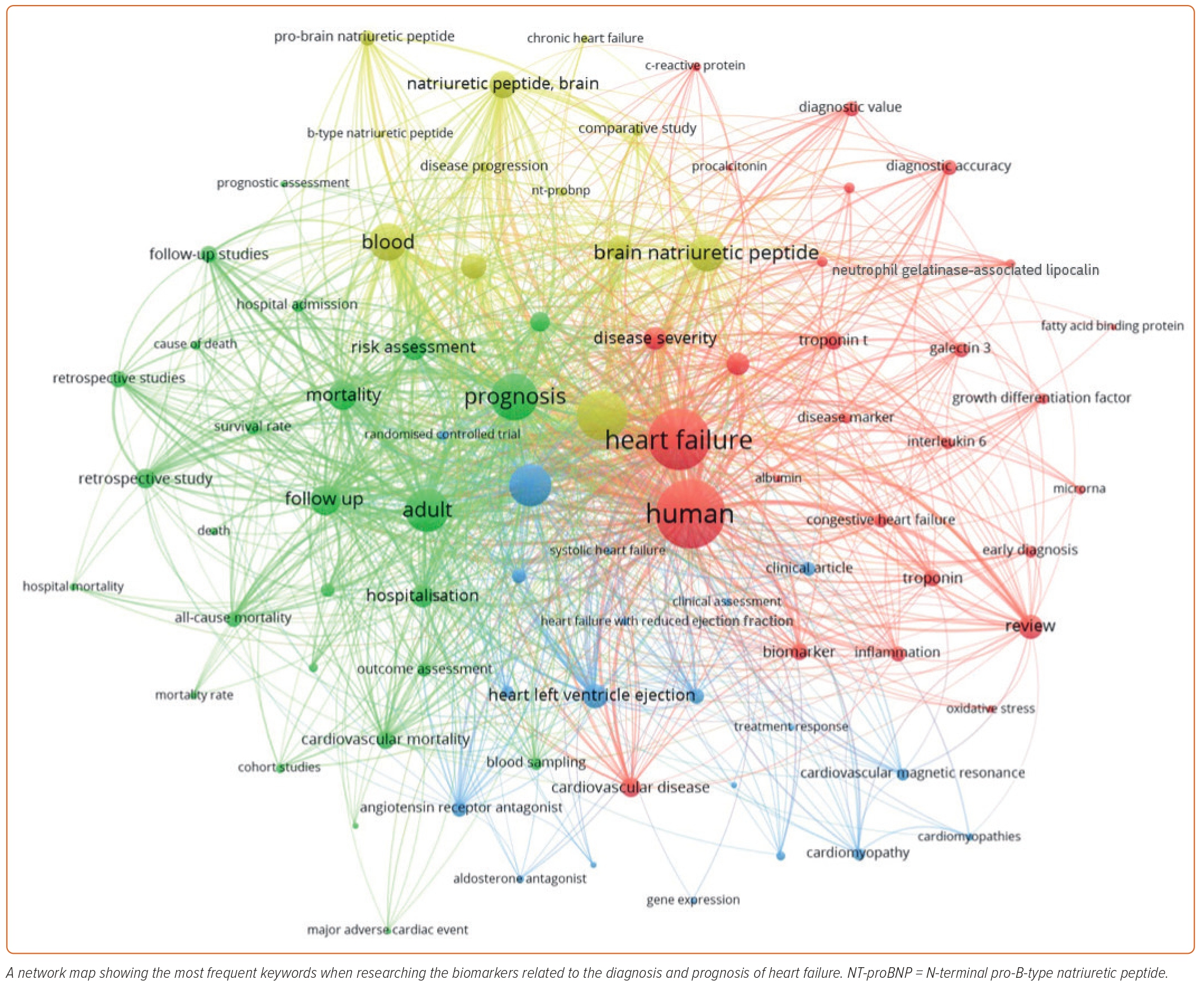
With the employment of the VOSviewer program (version 13), text mining was carried out to evaluate the co-occurrence networks related to the key terms ‘biomarkers,’ ‘heart failure,’ ‘diagnosis,’ ‘prognosis’ and ‘treatment response.’ These networks were visually presented with diverse analytical overlays, allowing a detailed exploration of the interconnections between the biomarkers and their multi-faceted uses in HF. For the analysis, the top 320 keywords with a minimum frequency of 20 were used as the foundation to derive primary themes, ensuring a robust examination of the role of biomarkers in HF. Following the selective filtering of relevant keywords, the final map was generated to depict the research hotspots within the research field vividly. The thickness of the lines connecting the different keywords in the generated map demonstrates the degree and extent of co-occurrence within the same relevant publications. This offers insight into the interrelation of the biomarkers’ roles (Figure 2).
Biomarkers to Diagnose Heart Failure
Cardiac Peptides
When the heart is under an excessive volume or pressure strain, ventricular cardiomyocytes, in particular, create BNP. Circulating BNP and NT-proBNP levels are usually low under normal conditions. However, as a compensatory strategy to return to normal haemodynamics, these levels considerably rise in people with HF.10
Researchers have made significant strides in developing diagnostic tools and identifying biomarkers for HF. A rapid diagnostic kit was made using recombinant NT-proBNP antigen. The kit demonstrated excellent reactivity, showing high specificity and its potential to assess HF risk early.11 Galectin-3 was also used in another study that showed higher sensitivity (94.3%), but lower specificity (65.1%) compared with BNP (77.1%) in diagnosing HF in patients with preserved ejection fraction. Additionally, the areas under the receiver operating characteristic curve for galectin-3 and BNP were 0.891 and 0.896, respectively.12
Another study assessed galectin-3’s potential in identifying and forecasting the incidence of HF with preserved ejection fraction (HFpEF) in a group at risk for HFpEF. With a sensitivity of 0.61 and specificity of 0.73 at a 13.57-ng/ml cut-off, galectin-3 surpassed the N-terminal pro-brain natriuretic peptide’s efficacy in identifying HFpEF. The adjusted composite of cardiovascular hospitalisation and death (p<0.001), adjusted all-cause mortality (p<0.001), and incident HFpEF (p<0.05) were also found to be predictably associated with greater galectin-3 levels. Galectin-3 has the potential to be used as an independent predictive marker in assessing cardiovascular events and mortality risk within this group at risk for HFpEF because these predictions were, crucially, independent of the influence of NT-proBNP.13
BNP levels were measured using a bedside assay in 1,586 patients with acute dyspnoea in the emergency department (ED). BNP levels alone were more accurate in identifying congestive HF (CHF) as the cause of dyspnoea than historical, physical or laboratory findings, with a diagnostic accuracy of 83.4%. BNP levels <50 pg/ml had a negative predictive value of 96%, effectively excluding CHF as the cause of dyspnoea. Additionally, when combined with other clinical variables, BNP measurements significantly improved the predictive power for diagnosing CHF.14
A study involving 1,256 patients aimed to establish broader standards for amino-terminal NT-proBNP testing in dyspnoeic patients with suspected acute HF. An age-related strategy using cut-points of 450 pg/ml, 900 pg/ml and 1,800 pg/ml for ages <50 years, 50–75 years and ≥75 years, respectively, achieved 90% sensitivity and 84% specificity in identifying acute HF. An age-independent cut-point of 300 pg/ml had a 98% negative predictive value for excluding acute HF.15 In the BACH trial involving 1,641 patients with acute HF (AHF) presenting with dyspnoea, mid-regional pro-atrial natriuretic peptide and mid-regional pro-adrenomedullin were assessed. Mid-regional pro-atrial natriuretic peptide proved non-inferior to BNP for diagnosing AHF, with an accuracy difference of 0.9%. The accuracy for predicting 90-day survival was 73% for mid-regional pro-adrenomedullin and 62% for BNP.16 The medical management of chronic HF has seen significant advances, and HF biomarkers, particularly BNP and NT-proBNP, are endorsed as gold standard markers for HF diagnosis and prognosis.
To rule out HF (class IIa, level of evidence C), the European Society of Cardiology’s recommendations advise using BNP and NT-proBNP. BNP and NT-proBNP reference values of <100 and 300 ng/l, respectively, are suggested for acute HF. The recommended reference values for chronic HF are <35 ng/l for BNP and <125 ng/l for NT-proBNP.2
Individuals arriving at the ED within 1 hour of symptom onset may test negative for cTn upon admission. In a study involving 28 acute MI (AMI) and 28 non-AMI individuals presenting to the ED within 1 hour of pain onset, blood donors were examined to establish a cut-off value for heart-type fatty acid binding protein (h-FABP). Among AMI patients, 55% tested positive for h-FABP, while 34.6% were positive for high-sensitivity troponin I (hs-TnI; p=0.015); notably, 21% were exclusively positive for h-FABP. The diagnostic accuracy was evaluated using a receiver operating characteristic curve, indicating that h-FABP exhibited higher sensitivity, but lower specificity than hs-TnI. The frequency of h-FABP positivity among AMI patients surpassed that of hs-TnI, which would have missed six cases. However, the area under the curve (AUC) for hs-TnI was superior to that of h-FABP. These initial findings suggest that h-FABP may be a promising candidate for rule-in/rule-out of AMI within the context of the ED.17
The association between pregnancy-associated plasma protein-A (PAPP-A) concentrations in coronary and peripheral blood, specific clinicopathological factors, and antioxidant enzyme activities was investigated in individuals diagnosed with coronary artery disease. Arterial blood was collected from 65 patients, and coronary blood was obtained via percutaneous coronary intervention. Spectrometric methods were employed to measure PAPP-A, catalase, superoxide dismutase-1 and superoxide dismutase-2 levels. Coronary PAPP-A levels were slightly higher than peripheral levels (81.25 ± 2.34 and 62 ± 3 ng/ml, respectively, p<0.0001), exhibiting a correlation between them (r=0.6629, p<0.001), but not with clinicopathological factors (p>0.05). Patients at risk for cardiovascular disease had significantly elevated coronary PAPP-A levels (p<0.05). Moreover, antioxidant enzyme activities were notably higher in coronary samples than peripheral samples in individuals with ischaemic cardiopathy secondary to atherosclerosis (p<0.001). However, neither coronary nor peripheral PAPP-A levels correlated with antioxidant enzyme activities in patients with cardiopathy secondary to atherosclerosis (p>0.05). These findings suggest that PAPP-A levels may be biomarkers for identifying individuals at risk of coronary artery disease.18
It was determined whether specific serum biomarkers could accurately diagnose chronic HF in patients who have known cases of chronic HF. These markers included BNP, cathepsin S, soluble ST2 receptor (sST2), platelet reactive protein-1 and interleukin-11 (IL-11). In patients with chronic HF, the levels of cathepsin S, platelet reactive protein-1, IL-11, sST2 and BNP were elevated, and substantially associated with the severity of cardiac dysfunction caused by chronic HF. As a result, the blood levels of cathepsin S, platelet reactive protein-1, IL-11, sST2 and BNP showed significant potential as independent indicators for detecting and diagnosing chronic HF.19
MicroRNA Family Members
Plasma levels of microRNA (miR)-302 family members, except for miR-302f, were significantly elevated in AHF patients. Among these miRNAs, miR-302b-3p demonstrated the highest AUC value of 0.87, indicating its strong potential as a diagnostic biomarker for AHF. Additionally, the levels of miR-302b-3p were significantly higher in AHF patients with left ventricular ejection fraction ≤45% and New York Heart Association (NYHA) class IV, compared to patients with left ventricular ejection fraction >45% and NYHA class II, respectively. This suggests that miR-302b-3p could also potentially help differentiate the severity of the disease.20
Plasma samples from 62 normal controls and 62 HF patients were analysed using real-time quantitative polymerase chain reaction. It was found that miR-21-5p, miR-30a-3p, miR-30a-5p, miR-155-5p, miR-216a and miR-217 showed different expression levels between healthy controls and HF patients, and their plasma levels were unaffected by haemolysis. Correlation analysis revealed strong correlations among these miRNAs, indicating the possibility of combined analysis. It was suggested that a combination of miR-21-5p, miR-30a-3p, miR-30a-5p, miR-155-5p, miR-216a and miR-217 could serve as a new diagnostic biomarker for early HF disease screening.21
The diagnostic utility of miR-208a was evaluated in detecting individuals with HF with reduced ejection fraction (HFrEF). The results showed that, in contrast to non-HFrEF patients, HFrEF patients had considerably higher levels of miR-208a (p<0.001). In terms of diagnosing HFrEF patients, the combination of miR-208a with NT-proBNP resulted in a considerably higher AUC value (0.83, 95% CI [0.76–0.90]) in contrast to NT-proBNP alone (0.73, 95% CI [0.64–0.82]). With rates of 68.0 and 90.2%, respectively, this combination also showed increased sensitivity and specificity.22
Serum exo-miRNAs (exo-miR-92b-5p, -192-5p and -320a) were analysed using quantitative real-time polymerase chain reaction. Exo-miR-92b-5p showed elevated expression levels in HFrEF patients compared with controls. It was inversely correlated with left ventricular fraction shortening and left ventricular ejection fraction, while positively correlated with left atrial diameter, left ventricular diastolic diameters and systolic diameters. A receiver operating characteristic curve revealed that exo-miR-92b-5p could discriminate HFrEF patients from controls with high sensitivity (71.4%) and specificity (83.3%). The findings suggest that elevated serum levels of exo-miR-92b-5p may be a diagnostic tool for HFrEF.23
Platelet miRNAs (pmiRNAs) as biomarkers for AMI remain uncertain, and the correlations between pmiRNA levels and platelet activity indices need to be well established. A study assessed the expression of platelet miR-1, miR-21, miR-126, miR-150 and miR-223 in 20 ST-segment elevation MI (STEMI) patients and 40 healthy volunteers. Platelet reactivity units were measured using a cartridge analyser, and vasodilator-stimulated phosphoprotein levels were determined by flow cytometry. No significant changes were observed in pmiR-1 expression. In STEMI patients, expressions of pmiR-21 and pmiR-126 were reduced, while pmiR-150 and pmiR-223 were elevated compared with controls (all p<0.01). Only pmiR-126 correlated with plasma cTn I (r=−0.556, p=0.011) in STEMI. However, there was no correlation between pmiRNAs and platelet reactivity units or vasodilator-stimulated phosphoprotein during admission or at 48 h post-stenting. Among the tested pmiRNAs, pmiR-126 emerged as a potential novel biomarker for STEMI, while pmiR-1, pmiR-21, pmiR-150 and pmiR-223 demonstrated limited usefulness. Additionally, as the assessed pmiRNA expression did not align well with platelet activity indices, their diagnostic potential appears constrained.24
Exhaled Breath Acetone
Exhaled breath acetone (EBA) levels were significantly higher in HF patients (median 3.7 μg/l) compared with the control group (median 0.39 μg/l). Among HF patients, those with acute decompensated HF (ADHF) had higher EBA levels (median 7.8 μg/l) compared with those with chronic HF (median 1.22 μg/l). The accuracy and sensitivity of EBA as a diagnostic method for HF and ADHF were approximately 85%, similar to BNP. Furthermore, the severity of HF, as classified by the NYHA, was associated with varying EBA levels, with increasing levels corresponding to higher HF severity.24
Receptor-interacting Protein Kinase-3
The protein kinase enzyme known as receptor-interacting protein kinase-3 is essential in controlling immunological responses, inflammation and programmed cell death. The diagnostic role of plasma receptor-interacting protein kinase-3 (RIP3) concentrations was assessed in 91 HF patients and 95 healthy volunteers, which revealed significantly increased RIP3 levels in HF patients compared with controls (p<0.001). The receiver operating characteristic analysis showed that plasma RIP3 is a potential diagnostic marker for HF with an optimal cut-off value of 357 pg/ml (sensitivity 84.6%, specificity 90.5%). The study suggests that plasma RIP3 could be a valuable diagnostic biomarker for HF.25
Genetic Hub Markers
Genetic hub markers are certain genetic variations or markers that play a crucial role in genetic networks or processes. Numerous genetic investigations, including network analysis and genome-wide association studies, are frequently used to identify these markers.
By performing bioinformatics network analysis using datasets from the Gene Expression Omnibus database, weighted gene co-expression network analysis identified vital modules, and Gene Ontology and Kyoto Encyclopaedia of Genes and Genomes pathway enrichment analysis highlighted platelet activation, chemokine signalling and focal adhesion as potentially involved in HF comorbid with depression. A protein–protein interaction network analysis revealed five hub genes: STAT4, CD83, CX3CR1, COL1A2 and SH2D1B. Invalidated datasets, STAT4 and COL1A2, were mainly associated with the comorbidity of HF and depression. These findings suggest potential new targets for mechanistic studies and treatment of HF and depression.26
Cardiac Myosin-binding Protein C
One essential regulatory protein in heart muscle cells, or cardiomyocytes, is cardiac myosin-binding protein C. It is essential for controlling the heart muscle’s contraction and relaxation, which improves the heart’s capacity to pump blood. A biomarker exclusive to cardiomyocytes, cardiac myosin-binding protein C may be capable of gauging cardiomyocyte damage even more precisely than high-sensitivity cTn.26–29 Patients with AHF had greater cardiac myosin-binding protein C concentrations at presentation than those with other final diagnoses (72 versus 22 ng/l; p 0.001).30
Biomarkers to Assess Heart Failure Severity
Cardiac Peptides
A valid predictive marker for chronic HF has been identified: sST2, a biomarker closely related to myocardial fibrosis and remodelling.31 The median follow-up length in a prospective cohort trial with 331 consecutively included patients with acute HF was 21 months. Patients with elevated sST2 levels had worse left ventricular ejection fraction, higher NYHA classification and higher levels of NT-proBNP. sST2 and NT-proBNP were shown to be independent risk factors for the primary outcome in all AHF patients by multivariate analysis. Additionally, there was a direct link between elevated sST2 levels and a rise in cardiovascular deaths.32 The frequently cited recognised prognostic threshold for sST2 is 35 ng/ml.33,34 According to Pascual-Figal et al., elevated sST2 levels are only prognostic when IL-1 levels are also raised.33 A further lower ideal threshold of 28 ng/ml has also been proposed in chronic HF.35
A different study examined patients with HFrEF and control subjects’ sST2 levels for diurnal change. It was discovered that for most subjects, peak sST2 levels mainly occurred during the day, especially around 5 pm, while the lowest concentrations were noticed at night, especially around 5 am. By using uniform sample times over repeated measurements, this temporal pattern may improve the diagnostic and prognostic usefulness of sST2. Notably, daily changes were absent in other biomarkers, such as NT-proBNP.36 These results are consistent with other research that has supported the steady trend of NT-proBNP throughout time.37
Inflammatory Markers
An investigation into the associations between IL-6, red cell distribution width and CRP and mortality in patients with HF was carried out in a prospective cohort study. It was discovered that all three indicators were connected to mortality. However, only IL-6 remained to be strongly related to mortality after comorbidities were taken into consideration. The optimal cut-off threshold for red cell distribution width, high-sensitivity CRP (hs-CRP) and IL-6 to predict mortality were 14.8, 68.7 and 52.9, respectively. It was noted that IL-6 had the highest specificity (75.35%) and sensitivity (100%).38 To determine their prognostic role in cardiovascular events, a study measured plasma pentraxin 3 (PTX3) levels in people with HFpEF. A greater risk of cardiovascular events was linked to higher PTX3 levels (>3.0 ng/ml).36 PTX3 levels were higher in chronic HF patients compared with healthy people with chronic HF. Higher PTX3 levels were associated with an increased risk of cardiovascular events in chronic HF patients (42% versus 0%).39
Two investigations, including individuals with HF, assessed the prognostic consequences of serum albumin levels. In the first study, acute decompensated systolic HF patients’ serum albumin levels were found to be a reliable predictor of death after 1 year. Comparing individuals with normal albumin levels and those with hypoalbuminemia revealed that patients with hypoalbuminemia had a higher 1-year mortality risk (37 versus 12%). One-year mortality was predicted with 70% sensitivity and specificity using a serum albumin threshold of 3.10 g/dl.40 Hypoalbuminemia was linked to poor survival rates (53%) and relatively higher amounts of cardiovascular deaths (84%) in patients with HFpEF.41
Two studies examined the possibility of the neutrophil:lymphocyte ratio (NLR) being used as a prognostic marker.42,43 The 3-year death rates for AHF patients in the highest NLR quartile were the highest during hospitalisation and after release.42 A cut-off value of 5.1 reliably estimated death throughout a 1-year follow-up period, with a sensitivity of 75% and specificity of 62%, and the NLR had an inverse relationship with left ventricular ejection fraction.43 Finally, NLR at baseline was examined as a potential predictive factor in a prospective cohort analysis of individuals with deteriorating or new-onset HF. Those with NLR in the highest tertile showed a significantly less favourable prognosis than those in the lowest tertile, despite the value of left ventricular ejection. Additionally, a drop in NLR at 6 months was linked to lower mortality rates. These results indicate that increased NLR may help identify patients with high-risk HF and may even be a therapeutic target.44
Neurohormones
When considering a combined outcome of all-cause mortality and readmission for HF, which includes patients with acute HF, baseline plasma renin activity (PRA) level at admission is a reliable predictive indicator. PRA also has incremental predictive value when NT-proBNP and clinically significant risk variables are added. The best threshold for predicting the combined outcome of all-cause mortality and HF readmission is 3.3 ng/ml/h for PRA.45 More recent research has emphasised the link between baseline PRA levels and the chance of 30-day HF rehospitalisation or death in patients with acute HF.46
Furthermore, in patients with acute HF who underwent treatment after receiving the most effective medical management, high PRA levels have been associated with mortality and rehospitalisation.47,48
In addition, even if left ventricular function is identical, greater endothelin-1 levels have been associated with worse HF outcomes, decreased right ventricular function, increased pulmonary pressure and increased left atrial volume index. The 75th percentile endothelin-1 value (5.90 pg/ml) is the ideal cut-off point for selecting the primary measure of time to the first cardiovascular event for multimarker modelling.49 The therapeutic significance of baseline PRA levels in predicting adverse outcomes, such as mortality and HF readmission in patients with AHF, is highlighted by these data. Additionally, increased endothelin-1 levels were linked to worse HF outcomes, and can be a valuable marker for anticipating cardiovascular episodes. The discovery of these biomarkers offers significant insights into prognosis evaluation and risk stratification in individuals with acute HF.
Oxidative Stress Markers
Chronic HF can mimic an oxidative disease, according to evidence from recent research.50–52 The link between oxidative stress indicators, disease severity and prognosis was examined in the setting of chronic HF related to ischaemic cardiomyopathy. Compared with controls, chronic HF patients showed higher lipid peroxidation (malondialdehyde) levels, plasma protein oxidation (reactive carbonyl derivatives and protein sulfhydryl groups), and lower glutathione peroxidase activity. It was determined that the risk of death was eightfold in individuals with malondialdehyde levels >8.0 mmol/l.53
Additionally, a lower serum-free thiol concentration, which indicates more significant oxidative stress, has been linked to worse outcomes and increased severity of HF among individuals with new-onset or worsening HF.54
These results highlight the likely involvement of the oxidative stress nature of chronic HF and its possible effects on the severity and prognosis of the disease. Creating specialised therapy approaches to lessen oxidative damage and enhance patient outcomes may be possible by comprehending the connection between oxidative stress and chronic HF.
Extracellular Matrix Markers
NT-proBNP and galectin-3 levels at baseline and over time were found to be related to specific changes in dyspnoea, echocardiographic remodelling and the composite outcome of cardiovascular death or HF hospitalisation in a cohort of patients with acute HF from sub-Saharan Africa. These results imply that these biomarkers may be helpful for the risk classification of AHF patients in sub-Saharan Africa and other regions with scarce resources.55 Additionally, it has been shown that the combination of NT-proBNP and galectin-3 can pinpoint those AHF patients who are most at risk for dying. In comparison with the sample with low levels of both biomarkers, patients with the highest quartile values for both had mortality rates as high as 15% within 10 days of presentation and a twofold higher 30-day mortality rate.56
Renal Markers
Both biomarkers, apelin-13 and angiotensin-converting enzyme 2 (ACE2), were found to be independent predictors of poor patient outcomes in a prospective study examining the prognostic significance of HF.57 It was discovered that a cut-off value >4,000.75 pg/ml, with a sensitivity of 87.5% and a specificity of 66.7%, was the best to forecast patient evolution when using ACE2. In contrast, the ideal cut-off value for apelin-13 was <402.5 pg/ml, with a sensitivity of 61.5% and a specificity of 76.9%.58 Previous experimental and clinical research found results that agree with these findings.59
Furthermore, a different study showed that when congestion and HF worsened, NT-proBNP levels increased, but apelin-13 levels decreased, validating these findings.60
Cystatin C’s predictive significance in acute HF was compared with other renal function markers and NT-proBNP. All-cause mortality at 12 months was significantly greater in patients with higher cystatin C levels, and mortality rates rose across cystatin C tertiles. Risk categorisation was further enhanced by combining NT-proBNP and cystatin C tertiles.61 Cystatin C and NT-proBNP were assessed for their potential role in prognosis in a prospective multicentre observational study of AHF patients with no renal dysfunction. A greater mortality risk of 37.8% was seen in patients with cystatin C levels >1.25 mg/dl, as opposed to 13.6% for those with levels below the cut-off. Cystatin C may perform better than NT-proBNP as a predictive marker in AHF patients with normal or mildly compromised renal function.62
These studies show that the predictive biomarkers for HF, ACE2, apelin-13 and cystatin C have promise. The capacity to forecast patient outcomes and direct treatment choices may be improved by incorporating these biomarkers into risk classification models.
A commonly tested and readily available renal marker that has shown to be valuable as a prognostic biomarker in HF is creatinine. Urinary creatinine significantly predicted prognosis in a study involving 2,130 patients. The study found that lower levels in the urine were linked to a higher NYHA class and, overall, a greater need for diuretics. A total of 31% of patients experienced the endpoint of hospitalisation for HF or all-cause death over a median follow-up period of 2.8 years.63 These findings are further supported by a different study that found an apparent association between decreased urine spot creatinine and a less favourable outcome in patients with new-onset HF or HF exacerbation.64
This relationship was further clarified by a prospective cohort research that included 108 acute HF patients treated at H Adam Malik Hospital between July 2018 and January 2019. Creatinine cut-off values of ≥1.7 mg/dl were established using receiver operating characteristic curve analysis, claiming that a creatinine level of 1.7 mg/dl was highly predictive of major adverse cardiovascular events during hospital stays, with a sensitivity of 87.5% and specificity of 79.5%. Multivariate analysis confirmed the status of creatinine ≥1.7 mg/dl as an independent predictor of major adverse cardiovascular events in patients with acute HF, highlighting its significance as a stand-alone predictor of adverse cardiovascular events during hospitalisation.65
To assess the prognostic effect of haemoconcentration on individuals with acute HF, a retrospective study was carried out on 188 enrolled patients and classified according to the degree of haemoconcentration. A primary parameter of haemoconcentration that was used in this study was creatinine, and the results show that it has a prognostic indicator with sensitivity and specificity. Further analysis determined the nature of the relationship between haemoconcentration and prognosis: patients with higher haemoconcentration had lower rates of mortality or rehospitalisation due to a cardiac complication.66
To summarise, the robust data indicating the prognostic importance of creatinine concentrations highlights its potential as a valuable biomarker for anticipating unfavourable cardiovascular events in patients with HF, providing essential information for improved clinical judgement and patient treatment.
Myocardial Injury Markers
The patterns of biomarkers, such as sST2, cTnT and cTnI, were evaluated in a study exploring diurnal fluctuation in individuals with HF.36,67 Based on the data, cTnT showed non-random diurnal variation, indicating swings throughout the day.36 Like cTnI, other biomarkers, such as NT-proBNP, did not fluctuate daily.68 Heart fatty acid binding protein (HFABP) was evaluated for its prognostic value in a prospective research involving HF patients with left HFrEF (ejection fraction 35%). HFABP concentrations were considerably more significant in HF patients than in control subjects, and they were associated with NYHA classes, and recognised biomarkers of cardiac dysfunction and remodelling, such as NT-proBNP, fibroblast growth factor 23 and galectin-3.69 This underlines the significance of sub-phenotyping HFrEF patients in creating individualised risk classification and therapy plans. Notably, the rise in HFABP has additionally been observed in cardiac failure with a normal ejection fraction.70 Another prospective study with patients who had acute HF discovered that cTnI may be more effective for predicting all-cause mortality than HFABP for long-term prognostication of AHF-related hospitalisation.71
These results highlight the potential utility of biomarkers, such as HFABP, cardiac troponins and sST2, in determining diurnal variation, risk stratification and prognosis in HF patients. Understanding these biomarkers’ dynamics can aid personalised care strategies and improve patient outcomes.
Exhaled Breath Acetone
Exhaled acetone was evaluated in two separate studies as a possible biomarker for HF diagnosis and prognostic value. The findings showed that the exhaled breath acetone (EBA) concentration of patients with ADHF was significantly elevated in comparison with those in the chronic HF group. It was noted that the median concentration of EBA in patients with ADHF was 7.8 g/l (interquartile range 3.6–15.2 g/l), while in the chronic HF group, it was 1.22 g/l (IQR 0.68–2.19 g/l).25 It was also determined that there was a direct relationship between the concentration level of EBA and the NYHA classification, which links it to severity and prognosis. A 1-year follow-up study on the same patients determined that a cut-off level of EBA concentration of >3.7 was associated with a mortality risk by 3.26-fold.72
Biomarkers to Assess Treatment Response
Plasma Biomarkers
Plasma biomarkers are molecules that can detect a disease’s presence, development or severity. They are found in the blood. Several plasma biomarkers have become well-known in the context of HF because of their capacity to offer insightful data regarding the underlying pathophysiological mechanisms and therapeutic response.73
BNP or NT-proBNP is one of the most well-known plasma indicators for HF. In reaction to increased ventricular wall stress and pressure overload, the heart releases BNP. BNP levels considerably increase as HF worsens, and monitoring these levels has become a standard method for identifying HF and determining its severity. BNP levels can also track a patient’s reaction to treatment. BNP levels that gradually decline show that the heart’s strain has decreased, signifying a favourable response to treatment. Using this information, clinicians can optimise patient outcomes by modifying drugs and therapies.74
Other plasma biomarkers, including endothelin-1, galectin-3 and hs-CRP, have additionally demonstrated promise in predicting therapy response and longstanding diagnosis in patients with HF. These indicators represent numerous pathological processes in HF, such as inflammation, fibrosis and endothelial dysfunction. The ability to track changes in these levels throughout treatment can help physicians better understand the efficacy of particular therapeutic interventions and direct them in developing a customised treatment plan for each patient.75
Natriuretic Peptides
The heart releases a series of hormone-like molecules known as natriuretic peptides in response to elevated pressure and volume burden. BNP and atrial natriuretic peptide (ANP) are the natriuretic peptides in HF that have been investigated the most. These peptides, produced in response to myocardial strain, are essential for controlling fluid balance and blood pressure.76
As was already established, BNP and NT-proBNP are commonly employed in clinical practice as biomarkers for diagnosing HF. They help observe therapy responses in addition to their diagnostic function. According to studies, a drop in BNP levels following the start of treatment is linked to better results and a lower chance of adverse events. In contrast, consistently elevated BNP levels despite treatment may signify a poor outcome, necessitating additional intervention or a change in the course of the patient’s care.77
ANP has also demonstrated promise as a biomarker for predicting treatment response in individuals with HF. Its levels can help detect patients more likely to benefit from particular medications, as they have been linked to changes in left ventricular function. Healthcare professionals can optimise therapy approaches and eventually improve patient outcomes and quality of life by including ANP measures in the treatment monitoring.78
Myocardial Injury Markers
A primary diagnostic tool for acute coronary syndrome, particularly in cases of MI or heart attack, has historically been troponin, a cardiac biomarker. Elevated troponin levels have been found in patients with HF, particularly those with a lower ejection fraction, highlighting the relevance of this finding beyond acute coronary syndrome.
Troponin readings have significant clinical ramifications for those with HF. First of all, increased troponin levels are a sign of myocardial injury, pointing to persistent cardiac damage and poor heart muscle function. Risk stratification is made more straightforward, and crucial information about the disease’s severity is provided. The increased risk of contrary cardiovascular events and higher mortality rates than patients with elevated troponin levels experience highlights the significance of early detection and targeted treatment in such circumstances.
Troponin levels have also become a crucial biomarker for evaluating how well HF patients respond to treatment. Troponin is a dynamic biomarker that enables clinicians to track alterations that reflect therapeutic therapies’ efficacy over time. Patients who initially have elevated troponin levels and then have a considerable drop in troponin levels after starting treatment are more likely to have improved clinical outcomes and a better prognosis overall. This demonstrates the significance of troponin as a tool for customising treatment regimens and making knowledgeable choices in managing individual patients.79
Novel Biomarkers for Heart Failure
MicroRNAs as Biomarkers for Heart Failure
MiRNAs are non-coding pieces of RNA that mainly help maintain RNA homeostasis. They are currently used as optimal biomarkers for the detection of HF due to their remarkable stability.80,81 Multiple studies have uncovered miRNAs’ diagnostic and prognostic role in recent years.
MiR-19b-3p was identified to have the most significant fold-change in a screening cohort among many other miRNA signatures. A validation cohort was then conducted to substantiate the prognostic value of miR-19b-3p by measuring its baseline level among patients with acute HF and following up for 19 months. Primary endpoints were hospital readmission due to HF or mortality due to any cause. It was found that the higher levels of miR-19b-3p at baseline were associated with worse survival; thus, indicating that miR-19b-3p could predict the occurrence of primary endpoints in patients with HF. Additionally, miRNA-19b-3p was found to have significant correlations with many proven cardiac biomarkers, such as serum sST2 (r=0.583), interventricular septal thickness (r=0.437), left ventricular posterior wall thickness (r=0.285) and left ventricular mass index (r=0.492).82
MiR-150-5p, among many other miRNAs identified in a screening cohort, was the most significantly associated with HF. A validation cohort was conducted after demonstrated dysregulation of miR-150-5p. MiRNA-150-5p was substantially downregulated in patients with advanced HF, as opposed to healthy controls and patients with mild-to-moderate HF, irrespective of the normalisation method used. miR-150-5p was significantly associated with maladaptive remodelling, severity of HF and outcome. Therefore, miRNA-150-5p could be a novel biomarker for patients with advanced HF.83
A case–control study aiming to identify multiple miRNAs as biomarkers for diagnosis and prognosis of acute HF reviewed miR-1, -21, -23 and -423-5-p. According to animal studies, miR-1 has been known to reverse cardiac hypertrophy.84,85 It has also been shown to be involved in atrial and ventricular arrhythmias, and has been theorised to play a role in acute coronary syndromes.86 MiR-21 has been linked to a profibrotic role within heart muscles by assisting inflammation, smooth muscle proliferation and fibrosis through the induction of MMP-2 production. MiR-23 upregulation has been associated with cardiac muscle hypertrophy.84–86 Although the study did not find a significant association between the miRNAs and prognostic outcomes, it did demonstrate that all the miRNAs studied did have diagnostic potential with relatively high sensitivity and specificity. MiR-1 was found to have the highest sensitivity and specificity (77.2 and 97.7%) for values >1.22. MiR-21 and MiR-23 were significantly lower in patients with HF due to ischaemia.
In addition, the investigation of the ZFAS1 gene/miR-590-3p axis’ role in determining the likelihood of CHF was assessed. The results showed that ZFAS1 gene expression was raised while miR-590-3p expression was diminished in CHF patients. These differential changes in ZFAS1 and miR-590-3p expression point to the possibility of their use as novel, non-invasive biomarkers for CHF diagnosis and prognostication. These encouraging findings could lead to better clinical outcomes and patient care in CHF.87
Insulin-like Growth Factor as a Biomarker for Heart Failure
Insulin-like growth factor (IGF) is associated with the activation and proliferation of smooth muscles in atherosclerotic plaques. This process is mediated by PAPP-A. This process leads to atherosclerotic plaque instability. PAPP-A is also involved in the proteolytic cleavage of IGF-binding protein-4, leading to the formation of the carboxy-terminal fragment of IGFBP-4 (CT-IGFBP-4).88 CT-IGFBP-4 has been recently found to provide increasing prognostic data in patients with STEMI regarding cardiovascular events (such as acute HF) and mortality.89–92
CT-IGFBP-4, CRP and NT-proBNP were all measured at baseline, and compared for patients with acute HF. After 1 year of follow-up, CT-IGFBP-4 weakly correlated with NT-proBNP and did not correlate with CRP, but it was more predictive of all-cause mortality than both biomarkers. The combination of all three biomarkers was significantly more predictive of mortality than any of the biomarkers alone; therefore, using all three biomarkers for the prognosis of acute HF should be recommended. Additionally, high levels of CT-IGFBP-4 were independently associated with 1-year mortality.88
Leucine-rich Alpha-2-glycoprotein-1 as a Biomarker for Diastolic Dysfunction
Leucine-rich α-2-glycoprotein-1 (LRG1) is a glycosylated protein that consists of many amino acids, especially leucine. LRG1 has been implicated in many disease processes, including inflammation and atherosclerosis.93 In an animal study, LRG1 was found to have a role in cardiac remodelling by regulating and inhibiting transforming growth factor-beta signalling cascades; thus, inhibiting cardiac fibrocyte activation and cardiac fibrosis.94 The study compared the levels of LRG1 in patients who presented with symptoms of chronic ischaemia, with half the patients having a clinical diagnosis of diastolic dysfunction. Plasma LRG1 levels were higher in patients with diastolic dysfunction, suggesting its role as a possible predictor of diastolic dysfunction.
Challenges of Using Biomarkers in Heart Failure
Lack of Standardisation
One of the primary challenges in using biomarkers for HF is the need for more standardisation across different assays and laboratories. Different laboratories may use varying methodologies to measure biomarker levels, leading to discrepancies in results. This lack of uniformity makes comparing data between studies and institutions complex, hindering the widespread adoption of biomarkers in clinical practice.95
Interference from Comorbidities
HF often coexists with other comorbid conditions, such as chronic kidney disease, diabetes or hypertension. These comorbidities can influence biomarker levels, complicating the interpretation of results. Distinguishing HF-specific changes from those related to other conditions becomes challenging and may require careful consideration of patient medical history and clinical context.96
Cost and Accessibility
Some biomarker assays can be costly, limiting their routine use, especially in healthcare settings with resource constraints. The availability and accessibility of advanced biomarker testing may vary across different regions and healthcare facilities, creating disparities in patient care and diagnosis.97
Limited Evidence for Clinical Decision-making
While biomarkers hold promise as diagnostic and prognostic tools, the evidence supporting their use in guiding clinical decision-making is still evolving. More research is needed to establish their precise role in tailoring treatment strategies and predicting patient outcomes. Integrating biomarkers into routine clinical practice requires robust clinical trials and validation studies.97
Confounding Factors
Specific biomarkers may be influenced by factors other than HF, such as age, obesity or renal function. These confounding factors can lead to false positive or false negative results, compromising the accuracy of biomarker-based diagnostics and prognostics.98
Limitations of Using Biomarkers in Heart Failure
Diagnostic Specificity
Despite their importance, biomarkers may not always diagnose HF definitively. Some biomarkers can be elevated in other cardiac or non-cardiac conditions, making distinguishing HF-specific changes solely based on biomarker levels challenging. Clinical judgement and a comprehensive diagnostic approach remain essential.99
Integration Into Clinical Practice
Incorporating biomarkers into routine clinical practice requires overcoming several hurdles. Healthcare providers need to understand the significance of specific biomarkers, their clinical implications and how to interpret their results accurately. Additionally, adequate infrastructure and resources must be in place for efficient biomarker testing and reporting.100
Dynamic Nature of Biomarkers
Biomarker levels can vary over time, reflecting changes in the disease status or response to treatment. This dynamic nature necessitates repeated assessments to ensure accurate monitoring and timely intervention.100
Ethical Considerations
Using biomarkers raises ethical concerns about patient privacy, informed consent, and potential implications for insurance coverage and employment opportunities. It is essential to ensure patient autonomy and confidentiality when using biomarkers in clinical settings.101
Standardisation of Cut-off Values
Establishing standardised cut-off values for specific biomarkers in HF remains an ongoing challenge. Determining clinically relevant thresholds ensures consistency and comparability across studies and patient populations.102
Discussion
Diagnostic Role
HF diagnosis integrates biomarkers that add novel and valuable information. Two of these biomarkers, BNP and NT-proBNP are particularly useful in identifying and evaluating the severity of HF, because they respond strongly to cardiac stress. They provide notably high sensitivity, specificity and negative predictive value, with BNP levels <50 pg/ml, effectively excluding CHF as the cause of dyspnoea. Although these biomarkers have been regarded as the gold standard for diagnosing HF, an increasing amount of evidence proves that combination with other clinical variables and biomarkers significantly enhances the predictive power for diagnosing CHF.9,14,16
Additionally, galectin-3 has been linked to fibrosis and inflammation in HF; thus, providing an essential role as a biomarker for diagnosing HF. During an assessment of individuals at risk for HF, especially in situations of HFpEF, high galectin-3 levels show potential, although having less specificity than BNP or NT-proBNP, but higher sensitivity in diagnosing HFpEF. Galectin-3 exhibits high sensitivity (94.3%), but comparatively lower specificity (65.1%) compared with BNP, making it a valuable tool for early identification.12
Alterations in the expression of miRNAs, such as miR-302b-3p, miR-21-5p, miR-30a-3p, miR-30a-5p, miR-155-5p, miR-216a and miR-217, have also been seen in individuals with HF. Exo-miR-92b-5p was found to have a sensitivity of 71.4% and specificity of 83.3% in discriminating HFrEF patients from controls, making it a novel potential diagnostic biomarker.23 Although considerably expensive, miRNAs can be an effective tool to diagnose HF, because they are readily detectable in the blood. However, there is limited information and evidence available on the impact of comorbidities on the levels of miRNAs, which suggests the need for further studies to be carried out before they can be heavily depended on as diagnostic markers.85
Moreover, when testing the role of miR-208a in diagnosing HF, the results demonstrated that miR-208a levels were higher in HF patients with HFrEF than non-HFrEF patients. Evidence also suggests that when using miR-208a in conjunction with NT-proBNP, diagnosing HFrEF patients was significantly improved than when NT-proBNP was used alone. Combining the two biomarkers also boosted sensitivity and specificity.22 Therefore, even though the role of miRNAs still needs to be explored, adding a commonly used and readily available biomarker supports and boosts the diagnostic role of novel biomarkers and can be implemented as a confirmatory tool.
Additionally, plasma RIP3 and EBA have emerged as novel and encouraging biomarkers. Acute decompensated HF is associated with an increase in EBA, which provides a non-invasive method of capturing metabolic changes related to the condition. Higher EBA levels were observed in patients with acute decompensated HF when compared with patients with chronic HF. Also, the accuracy and sensitivity of EBA were found to be very similar to B-type natriuretic peptide when used as a diagnostic method for HF and acute decompensated HF. Knowing that EBA has a sensitivity like the gold standard biomarker and is obtained through non-invasive measures solidifies its significance and potential role in being commonly implemented in clinical practice. Furthermore, increasing levels of EBA were associated with more severe HF, as classified by the NYHA.24
Moreover, RIP3 levels, which provide insights into inflammatory pathways, were significantly increased in HF patients compared with controls. Research showed that plasma RIP3, with an ideal cut-off level of 357 pg/ml, had high sensitivity and specificity (84.6 and 90.5%, respectively) to diagnose HF; thus, suggesting that plasma RIP3 has valuable potential to serve as a diagnostic biomarker for HF.25 Genes, such as STAT4, CD83, CX3CR1, COL1A2 and SH2D1B, serve as hubs that not only link HF and depression, but also shed light on the complex web of comorbidities that exists when gene expression and protein interactions are probed in greater depth.
Collectively, these indicators improve the diagnostic landscape of HF by providing doctors with a full arsenal for making accurate diagnoses, assessing severity, comprehending inflammatory processes and navigating potentially related illnesses. Collectively, the research cited above lends credence to the claim that many biomarkers, such as BNP, NT-proBNP, galectin-3, miRNAs, EBA, plasma RIP3 and hub genes, help diagnose HF, and improve clinical knowledge and decision-making.
Prognostic Role
The discovery of numerous biomarkers that provide distinct insights into the severity of the ailment and patient outcomes has greatly enriched the field of HF diagnosis and prognosis. Increasing evidence suggests that sST2, a measure of cardiac fibrosis and remodelling, is a strong prognostic predictor, with higher levels being linked to increased severity of HF and risk of cardiovascular mortality. An sST2 level of 35 ng/ml was frequently cited as the recognised optimal prognostic threshold for sST2.33,34 Additionally, there was a direct correlation between increased sST2 levels and a rise in cardiovascular deaths.32 Some research suggested a lower ideal threshold of 28 ng/ml in patients with chronic HF.35 Further evidence also proposed that elevated sST2 levels could only be prognostic if IL-1 levels were correspondingly raised.33
Predictive significance has also been found for inflammatory markers, such IL-6 and the NLR, highlighting the importance of inflammation in the development of HF. It was noted that IL-6 had the highest specificity (75.35%) and sensitivity (100%).38 Moreover, over a 1-year follow-up period, an optimal cut-off NLR level of 5.1 ng/ml was found to reliably estimate death in HF patients (sensitivity of 75% and specificity of 62%).103 Although the NLR can be easily and readily obtained from a complete blood count without requiring another blood sample to be taken, IL-6 has a higher sensitivity and specificity; thus, supporting the rationale of using IL-6 as a superior prognostic biomarker. Further evidence also fortifies the prognostic values of both IL-6 and NLR in HF patients.104,105
Research has also been carried out on plasma PTX3 to determine the prognostic role of cardiovascular events in HF patients with HFpEF. PTX3 levels >3.0 ng/ml were found to be associated with a greater risk of cardiovascular events.39
Cystatin C, a measure of renal function, and the neurohormones, apelin-13 and ACE2, have both been shown to have predictive value, especially with respect to mortality and subsequent hospitalisation. Studies proposed an optimal ACE2 cut-off value >4,000.75 pg/ml, with a sensitivity of 87.5% and a specificity of 66.7%, to evaluate HF prognosis. In contrast, the suggested ideal cut-off value for apelin-13 was <402.5 pg/ml, with a sensitivity of 61.5% and a specificity of 76.9%. Moreover, it has been shown that even with a comorbidity of mild renal failure, the predictive value of this biomarker remains unaffected and can, therefore, be implemented without any adjustments.58,59
Independent predictive information for cardiovascular events and mortality can be obtained from biomarkers related to extracellular matrix remodelling, such as galectin-3 and NT-proBNP. Indicators of myocardial injury, such as cTns and sST2, also have prognostic value. Knowing that troponins have a prognostic role benefits clinical judgement, as troponins are readily ordered in cardiac cases at presentation, which gives the value obtained a multifaceted use.25
Research has also investigated the prognostic role of galectin-3, as it is an effective diagnostic marker. Its additional role in prognostics further supports the rationale of using this marker in clinical practice.106 Additional evidence supporting the prognostic value of preoperative NT-proBNP level was studied in patients undergoing vascular surgery. A cut-off of 359 pg/ml−1 had a sensitivity and specificity of 73% each (AUC 80%; p<0.001) in predicting all-cause mortality, and sensitivity of 74% and specificity of 71% (AUC 75%; p<0.001) to detect a major adverse cardiac event. The overall 2-year survival rate was 84%; 93% in the <359 pg/ml−1 group and 68% in the ≥359 pg/ml−1 group (p<0.001). Following multivariate analysis, preoperative NT-proBNP at a value of ≥359 pg ml−1 remained an independent predictor of ACM (odds ratio 3.6; CI [1.6–8.1]; p=0.002). Postoperative NT-proBNP was a predictor of mortality, but not a major adverse cardiac event.105 Collectively, these indicators improve the knowledge of HF severity, patient outcomes and therapeutic approaches.
Treatment Response
The evaluation and management of HF therapy results have been greatly improved through the combination of multiple plasma biomarkers. Comprehensive information about the patient’s condition can be gleaned from the levels of several different markers, including BNP, NT-proBNP, endothelin-1, galectin-3, hs-CRP, ANP and troponin.74 High levels of BNP indicate a more severe form of HF. Monitoring BNP levels is useful for tracking HF progression and measuring therapeutic efficacy. A sustained drop in BNP levels after treatment is indicative of a favourable response and evidence of successful cardiac stress reduction. Additionally, consistently elevated levels of BNP despite treatment may signify a poor outcome or nonresponse to treatment, thereby forcing additional intervention or change in the course of the patient’s care.77
Endothelin-1, which is linked to endothelial dysfunction and vascular constriction, has been studied for its role in HF inflammation and vasoconstriction. Endothelin levels were also associated with treatment efficacy and warrant close monitoring.107 Further research affirms the potential role of galectin-3 as a treatment response indicator by establishing a link between galectin-3 levels and fibrosis and inflammation.108
Elevated levels of the inflammatory marker, hs-CRP, have been associated with an increased risk of cardiovascular disease. Reduction in inflammation, as measured by a drop in hs-CRP levels, is a positive indicator of therapeutic efficacy. Lower hs-CRP levels are also related to a potentially reduced risk of cardiovascular disease. Many studies highlight the importance of hs-CRP as an inflammatory measure.109 ANP, which the heart secretes when experiencing myocardial strain, helps regulate intravascular fluid balance and blood pressure. Therefore, monitoring ANP levels could predict treatment response, with changes in ANP levels suggesting improved left ventricular function due to treatment. Various research reaffirms the relevance of ANP in evaluating therapy efficacy due to its association with myocardial strain and fluid balance.110
To fully realise the potential of biomarkers in HF management, ongoing research and validation studies are necessary. As biomarker discovery continues to evolve, efforts towards standardisation, understanding the dynamic nature of biomarkers, and addressing ethical considerations will be crucial for their effective integration into clinical practice and enhancing patient care. Biomarkers are quantitative, low-cost and rational techniques for determining causal pathways that foretell unfavourable patient outcomes.
Late hospitalisation or early recovery biomarker tests are superior for predicting survival during the first 6 months after hospitalisation. If maximising post-discharge treatment and risk management in an outpatient context is the aim, then this implies that monitoring biomarkers upon hospital discharge and during the early post-discharge period could be desirable. Some of the most promising biomarkers, such as CRP, cTnI, IL-6, procalcitonin, PTX3, sST2, vascular endothelial growth factor receptor-1 and WAP four-disulphide core domain protein HE4, may require recurrent testing within 60–90 days of the first hospitalisation to maintain long-term prognostic accuracy.111 Ultimately, biomarkers hold the promise of revolutionising HF diagnosis, risk assessment and treatment, leading to improved patient outcomes and a reduced burden on healthcare systems.
Conclusion
Biomarkers offer valuable insights into HF diagnosis, risk stratification and prognosis. However, their practical implementation in clinical practice is hindered by challenges, such as lack of standardisation, limited evidence for decision-making and confounding factors. Addressing these challenges, and working towards standardisation and comprehensive validation will maximise the potential benefits of biomarkers in improving HF patient care and outcomes.