Transcatheter aortic valve replacement (TAVR) is now the preferred strategy for aortic valve replacement in most patients with severe symptomatic aortic stenosis.1,2 Occasionally, injury to the conduction system during TAVR results in high-degree atrioventricular block (HAVB) necessitating permanent pacemaker (PPM) implantation. Analysis from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry demonstrates stable rates of PPM implantation after TAVR from 2012 to 2015 of between 9 and 2%.3 Despite exponential growth in TAVR and improved operator experience, HAVB continues to be a complication which may necessitate PPM implantation.4,5 Recent expert consensus guidelines have suggested management strategies post-TAVR but comprehensive and prospective data on optimal risk stratification methods and management are lacking.6,7 Risk stratification tools to predict HAVB post-TAVR have been developed, but these are mostly based on data from single-centre studies and focus on the immediate post-TAVR setting.8–12
Little is known specifically about patients who develop HAVB after the initial hospitalisation for TAVR.13 With a trend towards a reduced length of hospital stay after TAVR and stable rates of post-TAVR PPM implantation, the presentation of HAVB has shifted towards the time after discharge from the index hospitalisation.14–16 Understanding patterns in timing of HAVB post-TAVR may help identify which patients may be at risk for this complication and help to mitigate adverse events. The goals of this review are to look at known risk factors for developing HAVB after TAVR, discuss trends in timing of presentation with HAVB after TAVR and explore the role of ambulatory monitoring in the management of HAVB after TAVR.
Risk Factors for Heart Block after Transcatheter Aortic Valve Replacement
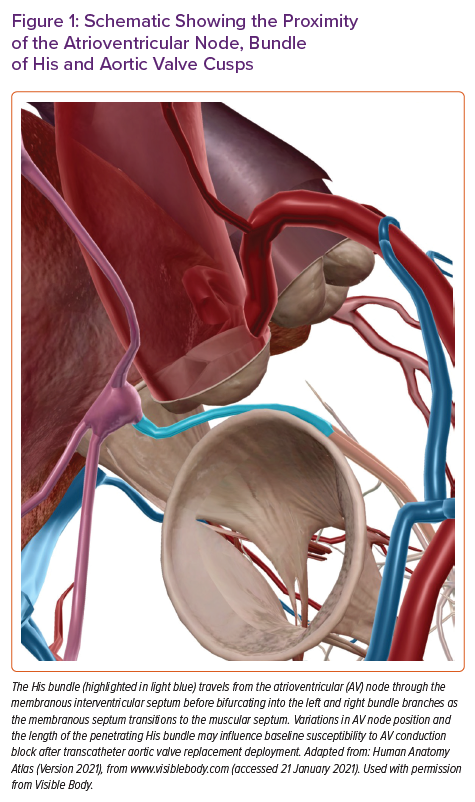
The relationship between AV block and deployment of TAVR prosthesis is driven by several anatomical considerations. The AV node and bundle of His lie in proximity to the aortic annulus. As the TAVR valve is inflated, there can be direct mechanical insult to the AV conduction apparatus from the AV node down to the left bundle branch itself. The penetrating bundle of His traverses the membranous intraventricular septum in the region of the commissure of the non- and right-coronary cusps (Figure 1). Variations in anteroposterior positioning of the AV node and the length of the penetrating bundle of His may influence a patient’s baseline susceptibility to post-TAVR HAVB after valve deployment.17 Additionally, pre-existing conduction abnormalities and procedural factors may influence the magnitude of injury to the conduction system. Various risk factors for post-TAVR HAVB have recently been reviewed, and while not a comprehensive list, this review highlights some of the more recognised risk factors, which can be divided into preprocedural and intraprocedural considerations.18
Preprocedural Factors
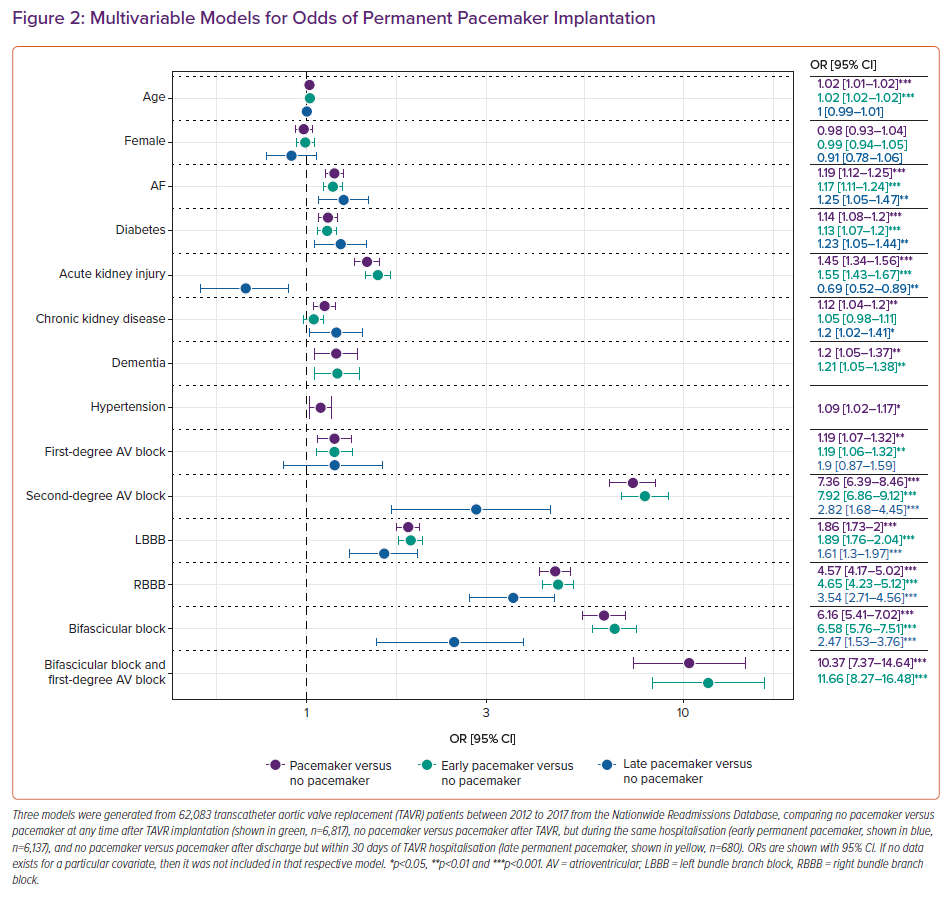
The most important risk factors for HAVB necessitating PPM implantation after TAVR are assessed in a preprocedural ECG. Specifically, pre-existing conduction defects, such as right bundle branch block (RBBB), left bundle branch block (LBBB), first-degree AVB, second-degree AVB and bifascicular block with or without first-degree AVB, have all been associated with an increased risk of HAVB after TAVR. Pre-existing RBBB represents one of the most significant risk factors.18 Deployment of the TAVR valve may injure the left bundle due to its proximity to the aortic annulus. Demographic and clinical characteristics that have been associated with post-TAVR PPM implantation include male gender and a history of AF.12,19,20 Our group previously analysed 62,083 TAVR patients from 2012 to 2017 from the Nationwide Readmissions Database in the US which further demonstrated that a history of diabetes, acute kidney injury, chronic kidney disease, dementia and hypertension were independently associated with PPM implantation within 30 days of TAVR (Figure 2).16 Pacemaker implantations post-TAVR were also stratified by whether they occurred early (prior to discharge from TAVR hospitalisation) or late (after discharge). Late PPM implantation after TAVR (versus no pacemaker implantation after TAVR) was also independently associated with a history of AF, diabetes and chronic kidney disease in addition to specific conduction abnormalities outlined later in this review. Acute kidney injury was less likely to be associated with late PPM than no PPM.
While specific conduction tissue anatomy can be defined with more detail as imaging techniques continue to develop, routine imaging and risk stratification based purely on conduction system anatomy needs further exploration.21 However, a simple 12-lead ECG to identify baseline conduction disease, in addition to patient-specific demographic and comorbidity characteristics provides valuable information to recognise patients who are at high risk of post-TAVR HAVB.
Procedural Factors
Transcatheter aortic valve protheses are deployed into the aortic valve annulus and secured into place using radial force applied to the native, often calcified, aortic valve. TAVR valves are deployed either by inflation and subsequent removal of an inner balloon over a wire or by an unsheathing technique which allows a controlled self-expansion into the annulus. Several procedural factors can influence the development of post-TAVR HAVB.18 The use of self-expanding valves over balloon-expandable valves, the degree of valve oversizing (greater than 15–20%) and lower implantation depth within the annulus have each been shown to increase the risk of HAVB after TAVR valve deployment.5,19,22–24 When a patient has been identified as having a high risk of HAVB, it may be warranted to use TAVR valves that convey lower risk for conduction defects.25
Timing of Heart Block
Immediate Onset
Transient HAVB is not uncommon with TAVR valve deployment and it is standard practice to have a temporary pacemaker wire in place both to rapidly pace the left ventricle to facilitate safe valve inflation with some implantation systems and as a safeguard in the event of HAVB developing. Most of this transient pacing requirement resolves on its own but it has also been shown to be associated with higher risk of the need for long-term pacing.12,26 Patients suspected to be in need of temporary pacing after the TAVR procedure should be considered for an internal jugular approach for the temporary pacing wire. This can improve patient comfort as observation periods with temporary pacing wires in place can go extend to 48 hours.7,12
Early Onset
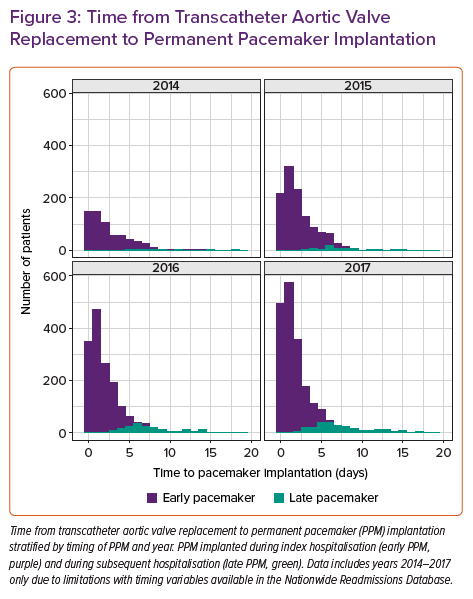
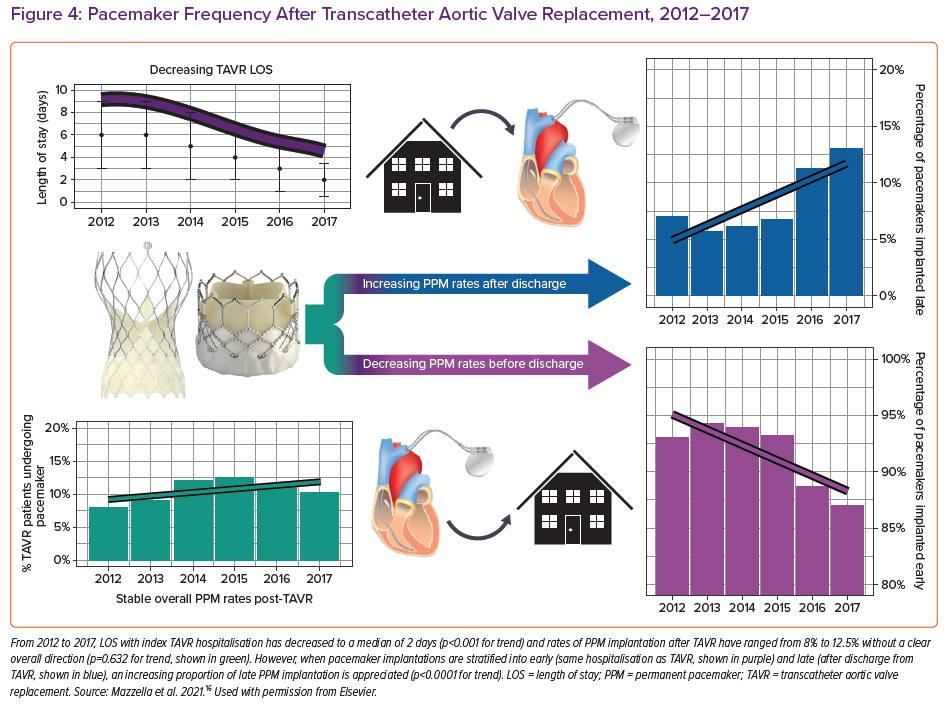
Several studies have reported that 60–96% of post-TAVR cases of HAVB occur within 24 hours of TAVR, while 2–7% occur more than 48 hours after TAVR.4,5 Length of stay after TAVR has decreased substantially over the past several years to a median of 2 days.14–16 This increases the possibility of HAVB occurring after a patient has already been discharged from hospital. Rates of PPM implantation within 30 days of having TAVR have remained stable at an average of 11% since 2012. Most of those pacemakers (90%) are implanted during the index hospitalisation, while the remaining 10% are implanted after the patient has been discharged. Early PPM implants (during index hospitalisation) occur at a median of 2 days post-TAVR – interquartile range (IQR): 0.5–3.5 days – while late PPM (during a subsequent hospitalisation) implants occur at a median of 7 days post TAVR (IQR: 5.3–8.7 days). Of the late PPMs, 79.6% were implanted within 14 days of TAVR. From 2014–2017, both the absolute and relative number of late pacemakers implanted after discharge from TAVR hospitalisation increased (Figure 3). This is likely to be related to a shortening of the length of hospital stay for TAVR combined with stable rates of PPM required during this time (Figure 4).16
Late Onset
Studies have described risk factors associated with the need for pacemaker implantation after TAVR. However, few have focused specifically on late-onset HAVB. The need for PPM usually occurs within 14 days post-TAVR and has been associated with an increased 3-year mortality.16,27 Mangieri et al. identified 54 out of 611 patients who underwent pacemaker implantation more than 48 hours after TAVR and found that baseline RBBB and an increase in PR interval after TAVR was associated with an increased risk of needing PPM, although it was unclear which patients were treated during the index hospitalisation or a subsequent stay after TAVR.28 Ream et al. demonstrated that RBBB was associated with delayed-onset HAVB at a median of 6 days after TAVR in 12 out of 113 patients monitored with a 30-day ambulatory monitor.29 Auffret et al. had similar findings in a multicentre sample of TAVR patients.30 Using ambulatory monitoring on discharge after TAVR, Tian et al. identified 11 out of 127 patients with delayed-onset symptomatic bradycardia and another nine patients with HAVB requiring PPM.31
Patients who develop late HAVB after discharge from TAVR hospitalisation represent a unique and growing group of patients at high risk for adverse complications of HAVB (Figure 4). These patients can present with syncope or sudden cardiac arrest which is particularly worrying in a frail and often elderly population who have undergone recent TAVR. In addition, there are significant healthcare costs associated with rehospitalisations in the postprocedural period. Increasing awareness of the potential risk for HAVB after discharge is important to prompt development of risk stratification and monitoring protocols. Several groups have implemented risk stratification scores to predict PPM implementation, although these scores do not discriminate early from late-onset HAVB.10,11
One area of growing research is the role of monitored outpatient cardiac telemetry to detect HAVB in real time so interventions can be enacted quickly.29,32 The relationship between AF and TAVR remains is not yet fully understood, although ambulatory monitoring has also been shown to detect novel AF after TAVR, allowing clinicians to consider starting therapeutic anticoagulation when appropriate.33 Some studies have also suggested ambulatory monitoring for conduction defects and arrhythmias prior to TAVR to increase the sensitivity in detecting risk factors for HAVB which may develop after TAVR.34,35
Late-onset HAVB that occurs more than 30 days after TAVR is a rare very high-risk phenotype. It becomes more difficult to directly link a TAVR procedure as a cause of PPM implantation as time passes. Patel et al. demonstrated a case of complete heart block 5 months after TAVR which was successfully treated with His bundle pacing.36 One theory about this degree of delay in presentation was subtle shifting in the valve over time, although the exact mechanism remains unknown.
Management of Heart Block After Transcatheter Aortic Valve Replacement
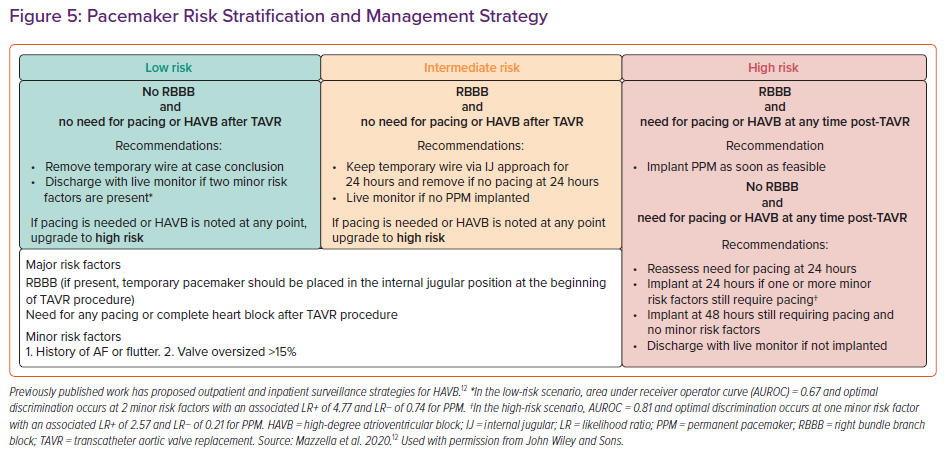
The 2013 European guidelines suggest a monitoring period of up to 7 days for resolution of HAVB after TAVR, while the 2012 American guidelines do not specifically address conduction defects after TAVR.37,38 Prospective randomised trials investigating surveillance for and management of HAVB after TAVR are lacking and current guidelines are based mostly on expert opinion.6,7 Our group has previously published our approach to conduction defects after TAVR, which primarily uses the presence of pre-existing RBBB and the need for immediate pacing requirement to provide risk stratification for patients and provides recommendations on duration of observation with temporary pacemaker wires in place (Figure 5).12 Some groups have also used intraprocedural rapid atrial pacing at the time of TAVR to uncover those with concealed intranodal conduction disease, as well as longer observation periods after TAVR using more durable screw-in temporary pacing systems.39,40 Recent expert consensus documents emphasise that electrophysiology studies (EPS) do not have a clear role in the risk stratification and management of heart block after TAVR.7 This is mostly due to the fact that trials investigating the use of EPS post-TAVR are either retrospective or lack a control group. Some studies have established some electrophysiological parameters, such as prolongation of the HV interval of ≥13 ms after TAVR, or induction of second-degree AVB when pacing the atrium at a rate of <150 BPM.41,42 Furthermore, existing bradycardia guidelines regarding indications for permanent pacing continue to apply.43
Approximately half of patients implanted with PPM after TAVR have greater than 40% pacing burden at a median of 4 years of follow-up.44 As a significant portion of patients regain intrinsic conduction after implantation, follow up and pacemaker programming should promote spontaneous atrioventricular conduction when possible.45 Irrespective of pacing burden after TAVR, it has been recognised that patients who require post-TAVR PPM implantation may be at higher risk of short- and long-term morbidity and mortality.44,46,47 These higher rates of adverse events may be a signal of frailty in an otherwise vulnerable population, an increased risk of complications from pacemaker implantation itself or other unknown causes. There are no consensus documents regarding device selection for pacing after TAVR. Percutaneous leadless pacemakers are of growing interest for people who have TAVR, particularly with the advent of devices which promote atrioventricular synchrony.48,49 Future studies are needed to assess the long-term outcomes of leadless pacemaker implantation for post-TAVR conduction defects. With the increased risk of mortality in the setting of PPM implantation and the additional risk of a secondary procedure, minimally invasive approaches may be particularly advantageous for the TAVR population.
Conclusion
Post-TAVR HAVB requiring PPM placement remains a relatively common complication. However, well-established preprocedural and procedural risk factors can be used to identify and advise high-risk patients and guide management. While most HAVB occurs within 48 hours of TAVR, there is a growing number of patients developing HAVB after initial TAVR hospitalisation due to the trend for early discharge post-TAVR. Several observation and management strategies have been proposed. As the population of patients at increased risk for late presentation of HAVB grows, development of algorithms for extended in-hospital observation or for mobile outpatient cardiac telemetry monitoring post-TAVR, particularly in the first 2 weeks after discharge, may be needed to reduce the risk of adverse events.