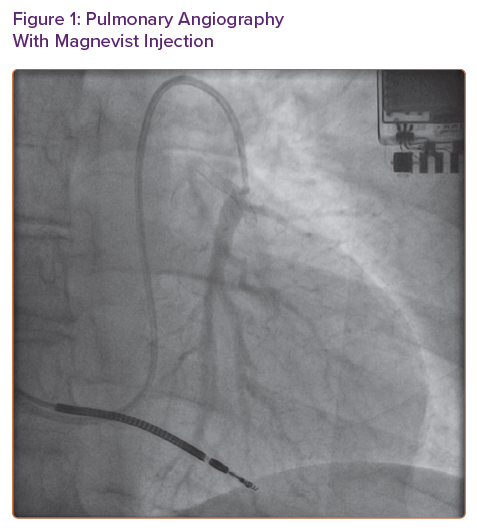
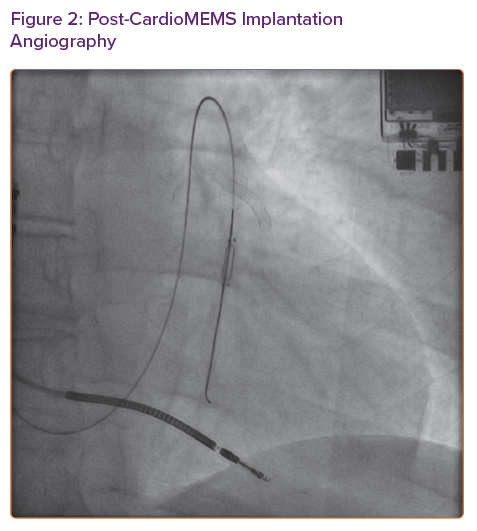
CardioMEMS is a wireless implantable pulmonary artery pressure monitoring device that has been shown to reduce heart failure hospitalisations in New York Heart Association (NYHA) Class III patients regardless of left ventricular ejection fraction.1,2 Since its approval in 2014 by the Food and Drug Administration, more than 5,500 devices have been implanted in the US.3 Implantation of the CardioMEMS device is an outpatient procedure including a right heart catheterisation to measure simultaneous right heart, pulmonary artery and pulmonary capillary wedge pressures and a limited pulmonary arteriogram using an iodine-based contrast agent (IBCA) to locate an appropriate target branch of pulmonary artery for device implantation.
Use of IBCAs exposes patients with advanced chronic kidney disease or iodine contrast allergy to significant risks, and a history of prior anaphylactic reaction virtually precludes their safe use. We report a patient with history of anaphylaxis from IBCA in whom substitution of a gadolinium-based contrast agent (GBCA) allowed safe implantation of the CardioMEMS device.
Case Report
A 57-year old woman with NYHA Class III heart failure with history of diabetes, hyperlipidaemia and hypertension presented with ischaemic heart failure with an ejection fraction of 40%. During the previous year she had three hospitalisations for decompensated heart failure and continued to struggle with volume management on outpatient basis; hence, she was referred for CardioMEMS implantation. Her allergy history included anaphylactic reaction to both shellfish and iodinated dye. On the day of the procedure, the patient’s serum creatinine was 1.13 mg/dl with an estimated glomerular filtration rate (eGFR) of 50 ml/min/1.73m2.
Because of concern for recurrence of her allergic reaction even if pre-treatment was given, we opted to trial GBCA (Magnevist) for limited pulmonary angiogram. We used 7 ml (0.09 ml/kg or 0.045 mmol/kg) of the contrast agent to obtain images (Figure 1). To facilitate pulmonary artery opacification, the contrast material was injected with balloon tip of the Swan-Ganz catheter inflated. Once a suitable sized branch vessel was identified, the CardioMEMS device was successfully deployed and calibrated in the room (Figure 2). The patient tolerated the procedure without any complications and was discharged home in stable condition.
Discussion
We describe, to our knowledge, the first successful deployment of CardioMEMS device using GBCA in a patient with history of anaphylactic reaction to IBCAs. Retrospective studies estimate the incidence of IBCA-related allergic reactions between 0.7% and 1.0 %, and the incidence of severe allergic reaction is estimated at 0.01%.4 Women and patients with history of asthma and autoimmune disorders generally have higher risk of IBCA-related allergic reaction, particularly in the setting of positive family history or known personal history of an allergy to shellfish.5 In patients with non-severe reactions, pre-medications with glucocorticoids and anti-histamines are recommended, although their efficacy is uncertain.5,6 Recommendations for patients with history of anaphylactoid reactions generally include avoidance of repeated exposure to IBCAs. Consequently, such patients may be denied access to implanted devices such as CardioMEMS and denial of their benefit to improve quality of life and decrease heart failure hospitalisations.
Several studies have evaluated the role of non-iodinated contrast materials such as those based on gadolinium and carbon dioxide in a variety of clinical scenarios.7 A review of gadolinium chelates for non-MRI applications found Magnevist to be the most extensively studied GBCA; a dose of 0.3–0.4 mmol/kg of ideal body weight has been recommended.8 It is worth noting that GBCAs used at doses higher than 0.4 mmol/kg have been associated with an increased risk of contrast-induced nephropathy and should be avoided among those with advanced renal disease (eGFR <30 ml/min/1.73 m2) or acute renal failure.9 However, in patients with severe allergic reactions to IBCAs such as our patient, GBCA may offer an attractive alternative to safely perform the implant procedure. Historically, one of the major concerns with GBCAs have been the risk of nephrogenic skin fibrosis, especially when eGFR is <30 ml/min/1.73 m2. However, contemporary evidence suggests this risk to be low (<1%) with newer generations of GBCAs.10
In our patient, using a much smaller dose of 0.045 mmol/kg, we were able to obtain good quality images and perform successful device implantation. In addition, our patient required no pre-medication and experienced no intra- or post-procedural complications.
Conclusion
This is the first report to describe successful use of GBCA in performing limited pulmonary angiography to implant the CardioMEMS device. In heart failure patients with history of severe reactions to IBCA and without severe renal dysfunction, the use of GBCA may offer safe alternative to guide implantation of the implantable haemodynamic monitoring system and could be considered when visualisation of a limited vascular area is required for implantation of other devices.