Heart failure (HF) is a highly prevalent disease in the community, with poor prognosis.1 Epidemiologically, approximately 50% of symptomatic HF patients have HF with reduced ejection fraction (HFrEF). The prevalence of HF has been estimated to increase by 46% by the year 2030, with the correspondingly large direct medical costs.2,3 Unfortunately, recent Medicare data suggest that 16.4% of patients with HF have had a potentially preventable readmission, implying that there is an opportunity to improve patient outcome, particularly for those with HFrEF, which can be treated with angiotensin-converting enzyme inhibitors (ACEis), angiotensin II receptor blockers (ARBs), angiotensin receptor neprilysin inhibitors (ARNis), mineralocorticoid receptor antagonists (MRAs), β-blockers and cardiac resynchronisation therapy to reduce risk.4
According to the CHAMP-HF (Change the Management of Patients with Heart Failure) registry, guideline-directed medical therapies (GDMT) for HFrEF were strikingly under-utilised. Notably, fewer than 30% of patients received GDMT at target doses, and only 1% were receiving ACEi/ARB/ARNi/β-blocker/MRA at target.5 Similar data were derived from both outpatient and inpatient registries.6
Additionally, of the GDMT, the ARNi sacubitril with valsartan (sacubitril/valsartan) was prescribed in only 13% of eligible patients, and at a target dose in 30% of these, even though the CHAMP-HF registry was published in 2018 and, hence, afterwards, the utilisation of GDMT and appropriate dosing of sacubitril/valsartan in clinical practice might have improved. Of note, there is a growing body of data on the efficacy and superiority of this compound, compared with other renin–angiotensin–aldosterone system (RAAS) blockers, which would clearly justify efforts to preferentially implement sacubitril/valsartan therapy in the treatment of HFrEF patients.7–14
Range of Use of Sacubitril/Valsartan in HFrEF Patients
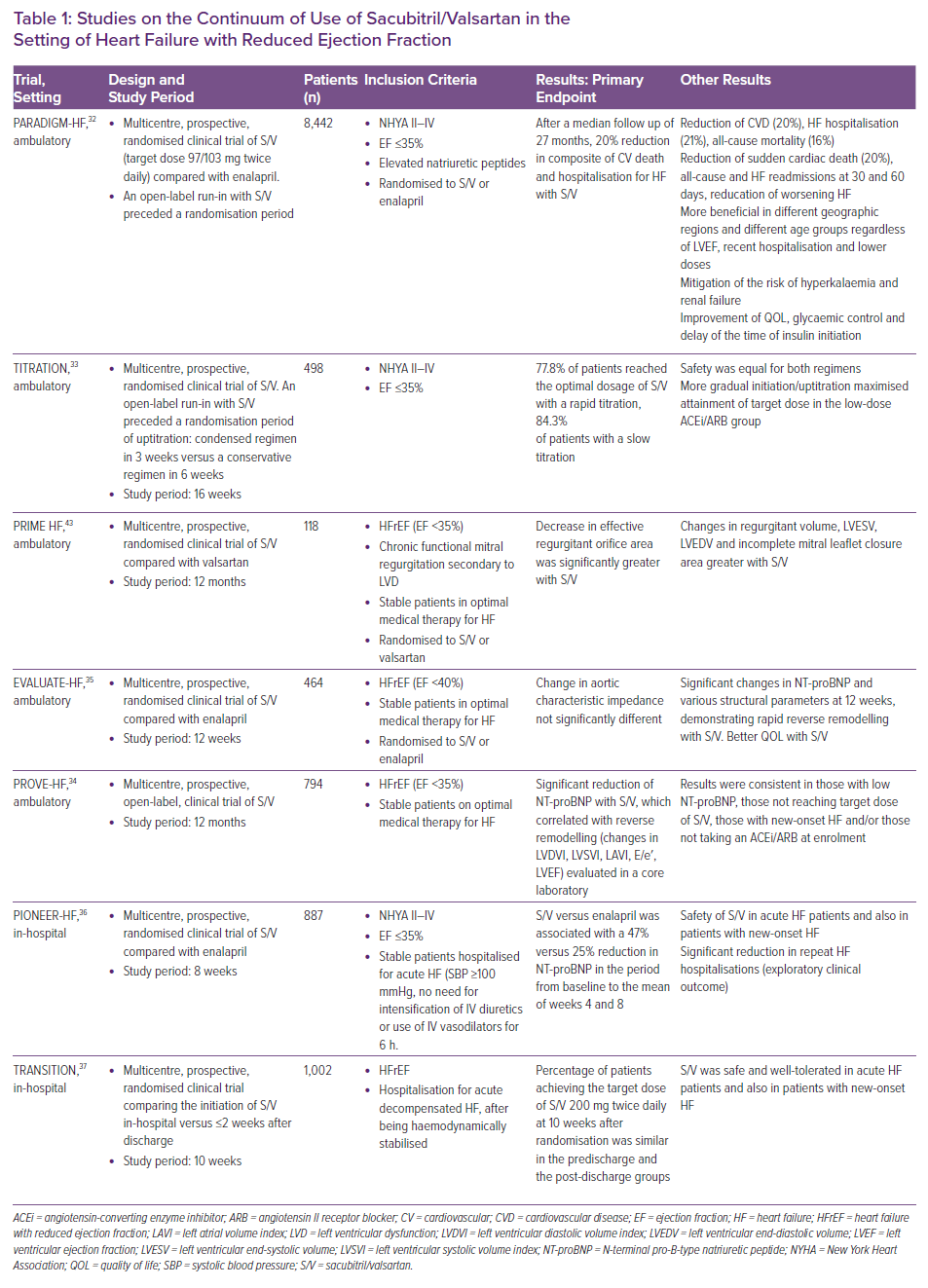
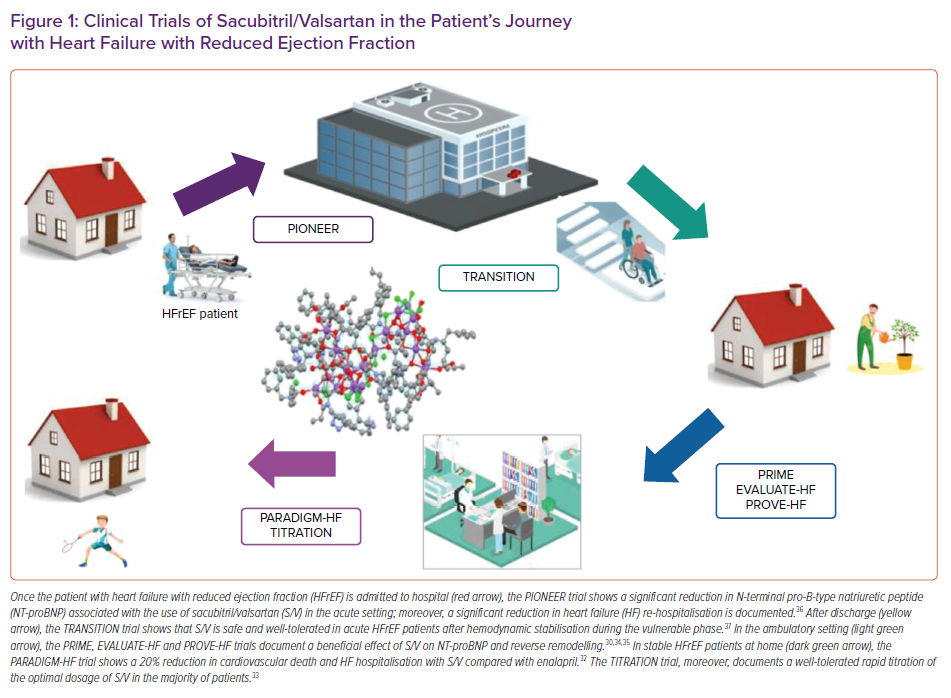
In the last few years, several studies have been published on the range of use of sacubitril/valsartan in the various settings of HFrEF.15,16 Importantly, these data derive not only from the landmark trial on the use of sacubitril/valsartan in HFrEF, the PARADIGM-HF, but also from studies, such as the TITRATION trial, on the possible modalities of titration of sacubitril/valsartan in clinical practice, the PIONEER and TRANSITION studies, which deal with the important topic of initiating sacubitril/valsartan in the acute HF setting, as well as the PRIME study, PROVE-HF and EVALUATE-HF studies, which provided insights into the reverse remodelling effect of sacubitril/valsartan (Table 1 and Figure 1 ).17–37
This plethora of data justifies the continuum of use of sacubitril/valsartan across the outpatient and inpatient settings, in the so-called ‘patient journey’, especially considering sacubitril/valsartan is not only beneficial but also cost-effective, according to three analyses recently published.38–41 This article will focus on this growing and convincing body of data and on the practical use of sacubitril/valsartan.
Studies in the Ambulatory Setting
PARADIGM-HF Trial
The PARADIGM-HF trial was a large (n=8,442) multicentre, prospective, randomised clinical trial of sacubitril/valsartan (target dose, 97/103 mg twice daily) compared with enalapril in patients with left ventricular ejection fraction (LVEF) ≤40%.32 After a median follow up of 27 months the trial was stopped early due to overwhelming clinical benefit of sacubitril/valsartan, with a significant reduction in the risk of cardiovascular death (including sudden cardiac death), and in HF hospitalisation; and the good safety profile. Although sacubitril/valsartan was associated with symptomatic hypotension more frequently than enalapril, more participants assigned to enalapril discontinued study medication due to adverse effects. Furthermore, even if the dose of sacubitril/valsartan was downtitrated from target because of hypotension, patients still had better outcomes compared with those on enalapril.
Based on the PARADIGM-HF trial results, sacubitril/valsartan is approved for use in patients with symptomatic HFrEF. However, limited information was provided from the PARADIGM-HF trial on how to initiate sacubitril/valsartan in clinical practice, when sacubitril/valsartan should be initiated (outpatient versus inpatient setting), whether sacubitril/valsartan can provide any meaningful and clinically relevant benefit on remodelling, and how the drug affects those not represented in the PARADIGM-HF trial (i.e. those with new-onset HF, those naïve to RAAS inhibition, those with lower concentrations of N-terminal pro-B-type natriuretic peptide [NT-proBNP] and those unable to be initially titrated to target dose). The results from several recently completed studies summarised in this article provide additional supporting evidence on the use of sacubitril/valsartan in HFrEF patients.
TITRATION Study
The TITRATION study was designed and conducted to provide guidance on how to initiate and uptitrate sacubitril/valsartan in those with chronic HFrEF. TITRATION enrolled 498 patients not previously on treatment, or with variable pre-treatment with ACEi/ARBs.33,42 Patients were randomised to one of two blinded arms: uptitration condensed, which included the uptitration of sacubitril/valsartan from 50 mg twice daily to 200 mg twice daily in 3 weeks including the run-in phase; and a conservative arm in which the titration from 50 mg twice daily to 200 mg twice daily was performed in 6 weeks, including the run-in phase. Treatment success, defined as tolerability of the drug, was achieved in 77.8% of the patients in the uptitration condensed arm, and in 84.3% of the conservative arm (p=0.078). TITRATION found that patients not on previous treatment with ACEi/ARBs or those on low doses of either may reach and maintain target doses of sacubitril/valsartan when the titration is more gradual. Additionally, in patients initially intolerant to the sacubitril/valsartan dose, a downtitration could be useful, allowing, eventually, for the target dose to be reached.
In summary, the TITRATION study demonstrates that sacubitril/valsartan may be titrated quickly, in 3 weeks, in most patients, except in ACEi/ARB-naïve patients or in those on a low background dose of ACEi/ARBs.
PRIME Study
This study was designed to provide evidence of a beneficial effect of sacubitril/valsartan on remodelling in HFrEF patients. In the PRIME study, the researchers conducted a double-blind trial of 118 patients with HFrEF and chronic functional mitral regurgitation secondary to left ventricular dysfunction (LVD) who were taking GDMT.43 Patients were assigned to valsartan or sacubitril/valsartan. Compared with the valsartan group, the sacubitril/valsartan had a greater remodelling benefit as manifested by: a greater reduction of effective regurgitant orifice area (−0.058 cm2 for S/V versus −0.018 cm2 for valsartan; p=0.032), which was the primary outcome of the study; and a greater decrease in regurgitant volume (mean difference, −7.3 ml; 95% CI [−12.6, −1.9]). Decrease in LV end-diastolic volume index was greater in the sacubitril/valsartan group (mean difference, −7 ml/m2; 95% CI [−13.8, −0.2]), while there were no significant differences in other left ventricular metrics, in incomplete mitral leaflet closure area, or in changes in blood pressure.
In summary, PRIME is a small study showing for the first time the reverse remodelling effect of sacubitril/valsartan in HFrEF with functional mitral regurgitation.
PROVE-HF Study
In the PARADIGM-HF trial, reduction in NT-proBNP concentration was tightly associated with improved outcome of patients treated with sacubitril/valsartan. Given that NT-proBNP reduction during GDMT has previously been linked to reversal of cardiac remodelling, the PROVE-HF sought to further examine this question. PROVE-HF was an open-label study of 794 patients with chronic HFrEF assigned to sacubitril/valsartan and evaluated on echocardiography prior to treatment, at 6 months, and at 12 months.34 NT-proBNP concentration was measured at each study visit. Following study completion, echocardiograms were transmitted to a core laboratory where they were interpreted following completion of all study procedures in a temporally and clinically blinded fashion. The study demonstrated a significant 37% reduction in NT-proBNP after initiation of sacubitril/valsartan; reduction in NT-proBNP was strongly associated with reverse cardiac remodelling. For example, from a baseline LVEF of 28%, by 12 months LVEF increased an average of 9.4%; many patients had even more dramatic improvement.34 In a similar fashion, there were decreases in indexed LV and left atrial (LA) volumes, LV mass index, and improvement in diastolic function as reflected in reduction of E/e′ ratio. Results were consistent between those with new-onset HF and/or those not taking an ACEi or ARB at enrolment (n=118 at baseline), or those not achieving the target sacubitril/valsartan dose (n=264).
In summary, the PROVE-HF study is more proof of the cardiac reverse remodelling process associated with sacubitril/valsartan, and of the significant reduction in NT-proBNP related to ARNi.
EVALUATE-HF Study
In EVALUATE-HF, the researchers assessed whether a change in aortic characteristic impedance might pathophysiologically contribute to the superiority of sacubitril/valsartan compared with enalapril in patients with HFrEF. They randomly assigned 464 patients with HF and LVEF ≤40% to sacubitril/valsartan or enalapril.35 At 12 weeks, the sacubitril/valsartan group had a decrease in aortic characteristic impedance (primary outcome) and the enalapril group had an increase in this parameter, but the difference was not statistically significant. However, the sacubitril/valsartan group had a significantly greater reduction in NT-proBNP, and greater reduction of several echocardiographic parameters, such as LV end-diastolic or systolic volume index, LA volume index, and mitral E/e′ ratio compared with the enalapril group (secondary endpoints).
Additionally, the investigators demonstrated a significant improvement in the overall summary score of the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ), an exploratory secondary endpoint. These data suggest a clear remodelling benefit even after 3 months of treatment with sacubitril/valsartan compared with standard care.
In summary, the EVALUATE-HF study, although demonstrating a non-significant improvement in aortic impedance with sacubitril/valsartan compared with enalapril, does demonstrate greater cardiac reverse remodelling and improvement in quality of life with sacubitril/valsartan (secondary endpoints).
Studies in the In-hospital Setting
PIONEER Study
Given that the PARADIGM-HF trial had excluded patients with acute decompensated HF, the PIONEER study was designed specifically to assess whether the superiority of sacubitril/valsartan versus enalapril was confirmed also in the acute setting.36 The enrolment of this trial was started as soon as the patients were haemodynamically stable during an inpatient HF admission. Haemodynamic stabilisation was defined as systolic blood pressure (SBP) ≥100 mmHg, no need for intensification of IV diuretics or the use of IV vasodilators 6 hours before randomisation. Patients were not allowed to have had IV inotropes in the previous 24 hours. In PIONEER, 881 patients were enrolled. One-third of patients had de novo HF, and 52% were not on an ACEi/ARB. Dosing was started with the lowest dose (24/26 mg sacubitril/valsartan twice daily or enalapril 2.5 mg twice daily) if blood pressure was between 100 and 120 mmHg. The median time to randomisation was 68 hours, and 25% were randomised in the first 48 hours. Then, based on blood pressure thresholds that changed through the trial, the dose was allowed to be uptitrated to the target (97/103 mg twice daily for sacubitril/valsartan versus 10 mg twice daily for enalapril). A total of 60% of the population reached the target dose of enalapril by 8 weeks, and approximately 55% reached the sacubitril/valsartan target dose.
Across the 8-week study period, sacubitril/valsartan treatment was associated with statistically significant NT-proBNP reduction compared with enalapril (47% versus 25%), which was the primary outcome of the study. There were no statistically significant differences in rates of symptomatic hypotension, worsening renal function, angioedema events, or hyperkalaemia between the two study arms (secondary outcome). Additionally, analysis of an exploratory clinical composite outcome (composite of deaths, rehospitalisation for HF, implantation of an LV assist device, or listing for transplantation), showed a statistically significant 46% RR reduction in favour of sacubitril/valsartan, entirely driven by a 44% reduction in repeat HF hospitalisations.
In summary, in the PIONEER study, the superiority of sacubitril/valsartan versus ACEi was confirmed also in the acute setting.
TRANSITION Study
TRANSITION was another study aiming to evaluate sacubitril/valsartan safety and efficacy in patients stabilised after hospitalisation for acute HF.37,44 The researchers randomly assigned 1,002 patients who were hospitalised for acute decompensated HFrEF to sacubitril/valsartan, after haemodynamic stabilisation. Patients were initiated on sacubitril/valsartan while still in the hospital or soon after discharge. Of the cohort, 29% were newly diagnosed with HFrEF and 24% had not previously taken ACEi/ARB. The primary endpoint, the proportion of patients achieving the sacubitril/valsartan target dose 97/103 mg twice daily at 10 weeks after randomisation, was achieved in 45% of the predischarge group and in 50.4% of the post-discharge group (RR ratio 0.893; 95% CI [0.783–1.019]). In addition, 86.4% of the predischarge group and 88.8% of the post-discharge group maintained any dose for at least 2 weeks before week 10 after randomisation (RR ratio 0.973; 95% CI [0.929–1.02]). Study drug discontinuation occurred in 4.5% of the predischarge group and in 3.5% of the post-discharge group (RR ratio 1.287; 95% CI [0.692–2.395]). Rates of adverse events, serious adverse events, and death did not significantly differ between the groups. Death rates were low and no deaths were related to the study drug, according to the researchers.
In summary, TRANSITION, similarly to the PIONEER study, showed the feasibility and safety of initiating sacubitril/valsartan in the acute HFrEF setting, even in new-onset patients. Given that the hospitalisation setting represents a pivotal moment in the clinical course of HFrEF and is associated with opportunities to fine-tune GDMT, data from these two trials provide reassuring information, and support ARNi initiation in this setting.
Sacubitril/Valsartan Therapy in Context
A common issue that arises for the clinician is the uncertainty about whether ACEi/ARB therapy is sufficient in their apparently stable patients with HFrEF. The studies summarised in this article,32–37,42–44 and the three meta-analyses, provide strong evidence for the superiority of sacubitril/valsartan, compared with conventional RAAS inhibition, in the outpatient setting.38–41,43,45–48 Importantly, the benefit of sacubitril/valsartan over enalapril was consistent, regardless of background therapy.27
Given that HF decompensation is the best clinical indicator of insufficiency of current HF treatment, available evidence prompts the substitution of an ACEi/ARB with sacubitril/valsartan also in this setting. Initiation during hospitalisation might allow for better titration and easier treatment of side-effects. The question of whether it might be better to start with an ARNi or an MRA in a de novo setting, would theoretically need formal testing. Of note, sacubitril/valsartan seemed to partially mitigate the risk of hyperkalaemia when the patient was already taking MRA, and long-term renal function seemed protected to a larger extent by sacubitril/valsartan compared with RAAS inhibitors.14 These data on renal protection with sacubitril/valsartan were consistent across the spectrum of LVEF.10,49–51
At the same time, recent data suggest that another class of drugs, the sodium–glucose co-transporter 2 (SGLT2) inhibitors (SGLT2i), should be added to the treatment for HFrEF.52,53 Whether ARNi/SGLT2i therapies should be implemented simultaneously or sequentially is unknown. However, there are promising data showing benefits in patients already taking ARNi who were randomised to dapagliflozin in the DAPA-HF trial.54 Additionally, it has been suggested that combining ARNi, SGLT2i, MRA and β-blocker therapy will lead to a significantly better prognosis in HFrEF.55
Finally, the use of sacubitril/valsartan in patients with higher LVEF, such as HF with preserved ejection fraction (HFpEF), is somewhat controversial. The PARAGON-HF study did show a borderline reduction in the combined primary endpoint of incident cardiovascular death or HF hospitalisation (p=0.059),56 and sensitivity analyses of this trial and of other HFpEF studies have shown that patients with LVEF between 40 and 55% might gain significant benefit from therapies such as ARNi.56,57 Thus, it might be worthwhile to distinguish the ‘curable’ HFpEF (i.e. with LVEF ≤55%), for which ARNi might prove to be effective through the antagonism of many pathophysiologic mechanisms of HFpEF, from HFpEF with LVEF >55%, which is normally associated with a significant burden of comorbidities, and which is, thus, not treatable to date.58–61
Practicalities of Sacubitril/Valsartan Use in HFrEF Patients
The patient with HF is characterised not only by advanced age but also by the presence of comorbidities, such as renal failure, hypotension and hyperkalaemia, which can represent a serious obstacle to the correct and effective implementation of GDMT. Nonetheless, the initiation and titration of sacubitril/valsartan is worthwhile, in order to promote reverse cardiac remodelling, improve symptoms and hopefully reduce the risk of cardiovascular events.
Clinicians contemplating the use of sacubitril/valsartan should consider several important steps. These include clear discussions with patients about the need for changing their treatment, and the steps needed to do so; inclusion of colleagues, such as advanced practitioners and other para-medical specialists, to assist in titration and follow-up and involvement of non-specialists in the decision-making and follow-up process.
Education
Shared decision-making and education are crucial when contemplating the use of sacubitril/valsartan, for several reasons. The therapy may be more costly than relatively inexpensive generic ACEis/ARBs and patients will need to understand the benefits of taking sacubitril/valsartan to reduce uncertainty. In those with acute HF, patients may also be unhappy about changing from medications they may have been taking for a long period of time; once again, a clear discussion about the advantages of sacubitril/valsartan relative to older GDMT is important. Patients should be taught about how the ARNi is initiated, and warned that hospital visits will be required to titrate the therapy. Education should be provided regarding the potential for hypotension and how to manage it, and patients should be warned about the very small risk of angioedema.
Initiation and Titration
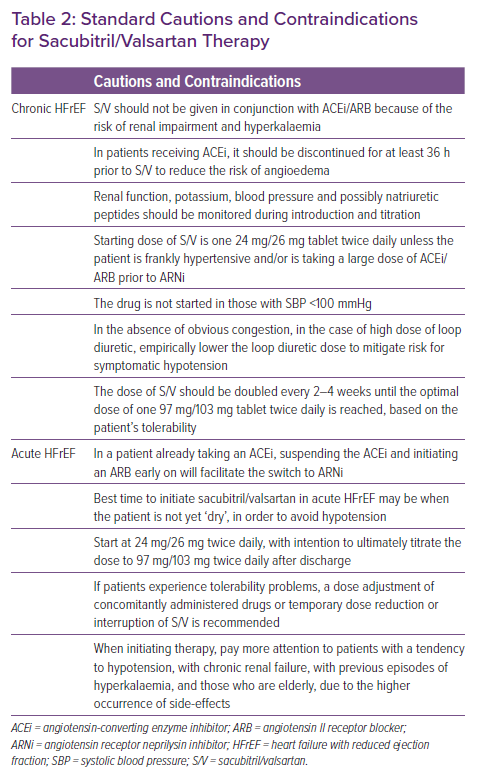
Standard cautions and contraindications for sacubitril/valsartan are listed in Table 2. If a patient is eligible for ARNi therapy, certain considerations are important prior to initiating and titrating sacubitril/valsartan. If a patient is receiving an ACEi, the drug should be discontinued for at least 36 hours to reduce the risk of angioedema. In the patient with chronic HFrEF, the generally recommended starting dose of sacubitril/valsartan is one 24 mg/26 mg tablet twice daily unless the patient is frankly hypertensive and/or is taking a large dose of ACEi/ARB prior to ARNi initiation. In the absence of obvious congestion, for those patients taking a high dose of loop diuretic, clinicians may choose to empirically lower the loop diuretic dose to mitigate risk of symptomatic hypotension. Reducing (or even discontinuing) the loop diuretic may be possible as the drug is titrated further. The dose of sacubitril/valsartan should be doubled every 2–4 weeks until the optimal dose of one 97 mg/103 mg tablet twice daily is reached, based on patient tolerability.26 This titration may be performed by members of the care team, including physicians, nurses, or pharmacists. It is reasonable to include monitoring of electrolytes, kidney function and possibly natriuretic peptides as sacubitril/valsartan is introduced and increased.
In patients with acute HFrEF, the indications, cautions, and contraindications for sacubitril/valsartan are similar. As with chronic HFrEF, if a patient is taking an ACEi, it must be discontinued 36 hours before ARNi initiation. If clinicians recognise that a patient taking an ACEi is likely to initiate sacubitril/valsartan later in their hospital course, suspending the ACEi and initiating an ARB early on will facilitate the switch to ARNi.
In the PIONEER study, patients with acute HFrEF were started on sacubitril/valsartan at a time when their diuretic dose was stable and was not being intensified.36 Given the rapid and significant effects on filling pressures, the best time to initiate sacubitril/valsartan in acute HFrEF may be at a time when the patient is not yet ‘dry’, in order to avoid hypotension. In acute HFrEF, it is suggested to start at 24 mg/26 mg twice daily, with intention to ultimately titrate the dose to 97/103 mg twice daily after discharge.33,42 It is critically important that at the time of discharge the patient has at least 30 days’ worth of sacubitril/valsartan and that insurance coverage has been confirmed, if applicable.
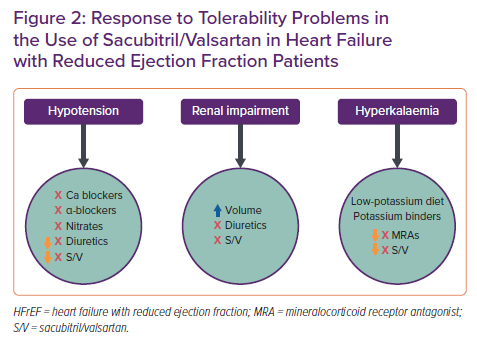
If patients experience tolerability problems (SBP ≤95 mmHg, symptomatic hypotension , hyperkalaemia, renal dysfunction), a dose adjustment of concomitantly administered drugs (e.g. reduction of furosemide during the starting of sacubitril/valsartan) or temporary dose reduction or interruption of sacubitril/valsartan are recommended (Figure 2).28 When initiating therapy, it is necessary to pay more attention to patients with a tendency to hypotension, with chronic renal failure, with previous episodes of hyperkalaemia, and those who are elderly, because these are the patients in whom the appearance of side-effects is more frequent.
Special Circumstances
Renal Impairment
- No dose adjustment of sacubitril/valsartan is required in patients with mild renal impairment (estimated glomerular filtration rate [eGFR] 60–90 ml/min/1.73 m2).
- In patients with moderate renal impairment (eGFR 30–60 ml/min/1.73 m2), an initial dose of sacubitril/valsartan 24/26 mg twice daily should be considered.
- In patients with severe renal impairment (eGFR <30 ml/min/1.73 m2), sacubitril/valsartan should be used with caution and a starting dose 24/26 mg twice daily is recommended, given that in this patient setting clinical experience is very limited.
- In patients with end-stage renal disease the use of sacubitril/valsartan is not recommended because there is no clinical experience.
The use of sacubitril/valsartan may be associated with a decrease in renal function, especially if dehydration or concomitant use of non-steroidal anti-inflammatory drugs is present. Dose reduction should be considered in patients who develop a clinically significant decrease in renal function. Evaluation of patients with HF should always include examination of renal function, given that patients with mild–moderate renal impairment are more at risk of developing hypotension.
Hypotension
Treatment with sacubitril/valsartan should be initiated in patients with SBP ≥100 mmHg. Given that cases of symptomatic hypotension have been reported in patients treated with sacubitril/valsartan in clinical trials, especially in patients ≥65 years of age, in patients with renal disease, and in patients with low SBP (<112 mmHg), when starting therapy or during titration of the sacubitril/valsartan dose, blood pressure should be routinely monitored. Routine assessment of blood pressure is done for all vasoactive therapies commonly used for the condition of HF. If hypotension occurs, a temporary dose reduction or withdrawal of sacubitril/valsartan is recommended. A dosage adjustment of diuretics, concomitant antihypertensives, α-blocker drugs used for the treatment of prostatic hypertrophy and for other causes of hypotension (e.g. hypovolemia) should also be considered.
Hyperkalaemia
Treatment with sacubitril/valsartan should not be started if the serum potassium level is >5.4 mmol. Given that sacubitril/valsartan may be associated with an increased risk of hyperkalaemia, monitoring of serum potassium is recommended, especially in patients who have risk factors such as renal impairment, diabetes, hypoaldosteronism, or are on a high-potassium diet or treated with MRAs. If patients experience clinically relevant hyperkalaemia, teaching about low potassium diets is the first step. If hyperkalaemia persists, the dose adjustment of the concomitant medication or temporary dose reduction or withdrawal is recommended. The use of potassium-binding agents to facilitate use of sacubitril/valsartan may also be considered.
Conclusion
Clinical practice guidelines for HFrEF recommend that patients be stable on an optimal dose of ACEi/ARB before sacubitril/valsartan implementation, whereas the regulatory labelling for sacubitril/valsartan in the EU and US is more liberal.62,63 In fact, it does not require any specific dose of ACEi/ARBs or even prior ACEi/ARB treatment at all. In light of recent data from studies subsequent to PARADIGM-HF, it seems compelling and obvious that initiation of sacubitril/valsartan is preferred regardless of pretreatment with ACEi/ARB, as conceded by consensus documents published by the American College of Cardiology and European Society of Cardiology.64,65 Eligibility for treatment would largely follow the regulatory labelling for sacubitril/valsartan, namely symptomatic HFrEF without contraindication, the inclusion criterion used in the PROVE-HF study. Besides clear benefit in ACEi/ARB-naïve patients, the results from PROVE-HF suggest that more nuanced means to identify eligible patients should be avoided: for example, prescribing ARNi only for those with elevated NT-proBNP or only for those who might tolerate maximum doses is not advisable.34