Heart failure (HF) remains one of the most common medical conditions worldwide, placing a continuously growing burden on healthcare providers. Within the HF population itself, the subset of patients who develop pulmonary hypertension (PH-LHD) has been identified as having a significantly higher morbidity and mortality.1 There are limited therapeutic options for PH-LHD and it often complicates the use of standard treatment approaches. This article will focus on PH-LHD as it relates to patients with both HF with reduced ejection fraction (HFrEF) – left ventricular ejection fraction (LVEF) <40% – and HF with preserved ejection fraction (HFpEF) – patients with LVEF >50%.
Definition
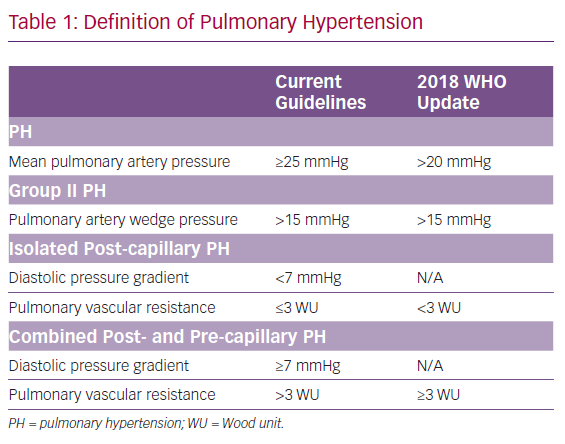
Previously, PH was defined by a mean pulmonary artery pressure (mPAP) ≥25 mmHg with PH-LHD; with the WHO defining group II PH as mPAP ≥25 mmHg in the setting of a pulmonary artery wedge pressure (PAWP) >15 mmHg.2 There is a continuum of disease comprising PH-LHD pathophysiology and several haemodynamic variables have traditionally been incorporated into the definition of PH-LHD to differentiate between these two subgroups. These variables include the diastolic pressure gradient (DPG), which is defined as the difference between the diastolic pulmonary artery pressure and the PAWP; the transpulmonary gradient (TPG), defined as the mPAP–PAWP; and pulmonary vascular resistance (PVR), defined as the TPG divided by the cardiac output.
According to the 2015 European Society of Cardiology and the European Respiratory Society guidelines for the diagnosis and treatment of PH, isolated post-capillary PH (IpcPH), is defined as PH-LHD with DPG <7 mmHg and/or PVR ≤3 Wood units (WU), and represents the majority of PH-LHD, with the predominant causative factor being elevation in left-sided pressures. By comparison, combined post- and pre-capillary PH (CpcPH), the group previously referred to as having out-of-proportion or reactive PH-LHD, with a prevalence of 12–38%, was defined as PH-LHD with DPG ≥7 mmHg and/or PVR >3 WU.2,3
In 2018, the 6th World Symposium on Pulmonary Hypertension recommended changing the definitions of PH, with the goal of identifying patients with earlier stages of PH who could potentially benefit from interventions. This recommendation was to define precapillary PH as mPAP >20 mmHg in the setting of an elevated PVR.4 The rationale for this change was based on previous studies that found a mPAP cut-off of 20 mmHg is two standard deviations above normal mPAP value.5,6 The group also suggested updates to the definitions of PH-LHD; IpcPH was defined as mPAP >20 mmHg, PAWP >15 mmHg and PVR <3 WU. CpcPH was defined as mPAP >20 mmHg, PAWP >15 mmHg and PVR ≥3 WU. The rationale for a change away from DPG to PVR exclusively included concern for the fidelity and interpretation of the DPG measurement.4,5 A summary of these changes is presented in Table 1.
Prevalence, Prognosis and Pathophysiology
PH-LHD is remarkably common, accounting for 65–80% of all PH patients, with the prevalence of PH estimated at 40–75% in people with HFrEF, and 36–83% in people with HFpEF.3,7–10 PH is a poor prognostic indicator in all HF patients, with PASP >45 mmHg on echocardiography being associated with increased 5-year mortality, independent of the severity of HF and other comorbidities.11,12
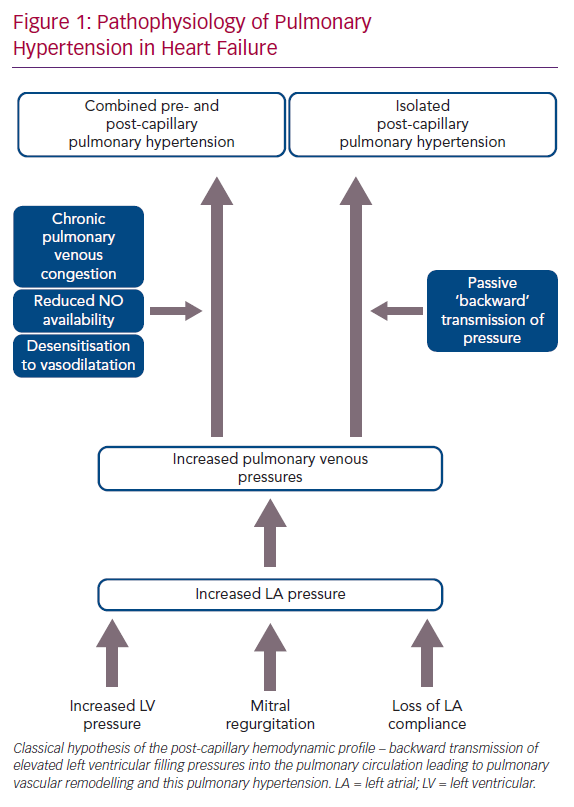
The pathophysiology of PH-LHD is thought to be a continuum, where the initial transmission of elevated left-sided filling pressures into the pulmonary circulation is followed by superimposed components, such as pulmonary vasoconstriction, decreased nitric oxide availability and desensitisation to natriuretic peptide-induced vasodilatation. This process leads to pulmonary vascular remodelling including thickening of the alveolar-capillary membrane, medial hypertrophy, intimal and adventitial fibrosis and small vessel luminal occlusion (Figure 1).3
More recently, Fayyaz et al. studied pulmonary arterial and venous remodelling in autopsy specimens from patients with PH-HFpEF and PH-HFrEF compared with normal controls and those with pulmonary veno-occlusive disease (PVOD). They found that more venous intimal thickening was present compared with arterial intimal thickening in those with PH-LHD, and this was similar to changes seen in people with PVOD. These changes correlated with PH severity, suggesting that the pulmonary venous remodelling promoted and dictated the development and severity of PH in the HF population.13 Additionally, recent work has further assessed the impact of left-sided valvular disease on PH, with nearly 50% of patients with severe aortic stenosis having PH, of whom 12% had CpcPH, which was associated with higher PAWP, lower pulmonary arterial compliance (PAC) and was a significant predictor of mortality.14
Diagnosis
Echocardiography
Echocardiography is one of the mainstays of investigation of LHD in general and efforts have been made to diagnose and monitor PH-LHD using routine echocardiography. This has been well summarised in a recent review by Maeder et al.9,15,16 Pulmonary artery systolic pressure (PASP), the most well-known parameter, can be estimated by measuring peak tricuspid regurgitation velocity, applying the modified Bernoulli equation (4v2) and adding estimated right atrial pressure (most commonly using inferior vena cava size and collapsibility).17–19 Studies have shown a good correlation with invasive haemodynamic measurements, although PASP estimates often have reduced accuracy due to: the technical ability required to acquire quality images; problems with tricuspid regurgitation velocity (low, absent or of poor quality or with severe tricuspid regurgitation); or when right atrial volume is unable to be assessed or is inaccurately estimated.20 Additionally, PASP alone cannot determine the underlying haemodynamic PH phenotype.21 Therefore, other more reliable and informative measures for assessment have been evaluated for the PH-LHD population.
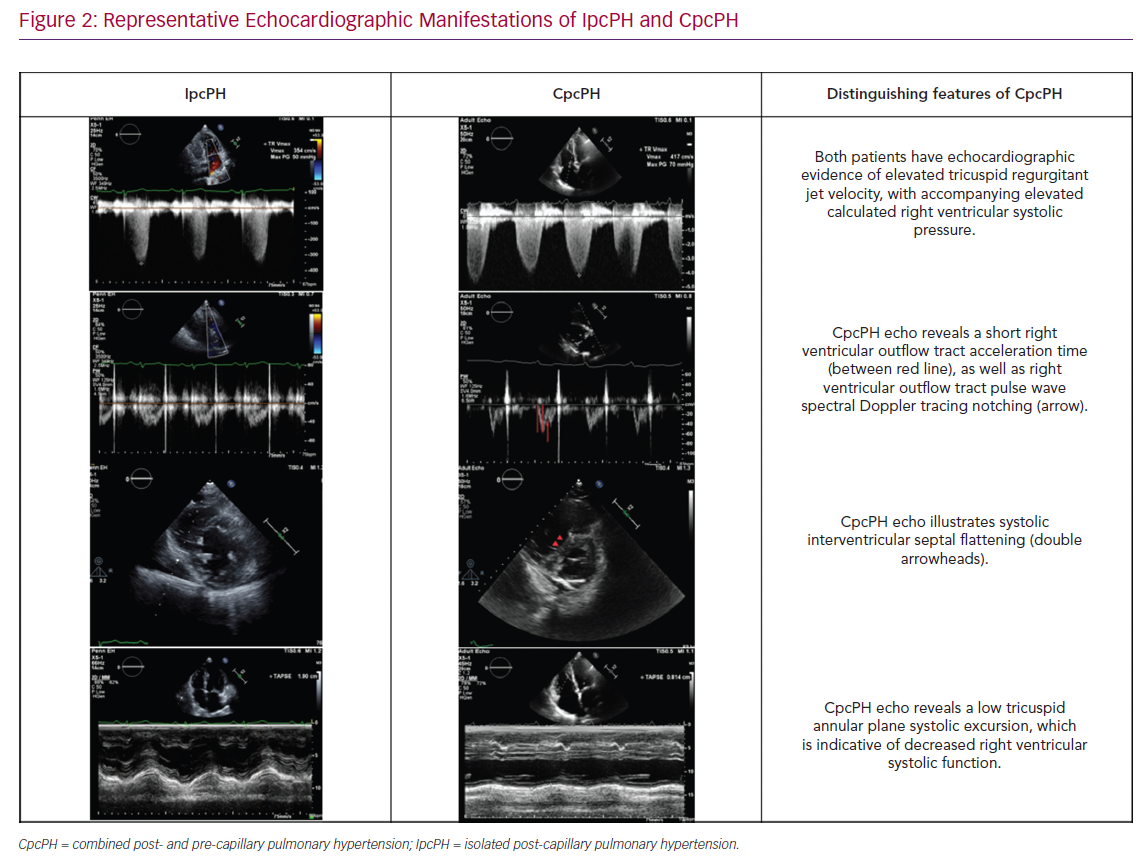
There has been a focus on assessing the RV–PA interaction and/or afterload elevation in people with PH-LHD. This includes the assessment of septal flattening (particularly in systole), RV dilatation, RV to LV ratio, RV apex angle and RV systolic impairment (as measured by RV fractional area change or tricuspid annular plane systolic excursion (TAPSE) and RV longitudinal strain (as measured by 2D and 3D speckle tracking) (Figure 2).22 Furthermore, the right ventricular outflow tract (RVOT) pulse wave Doppler profile contains several parameters to inform the underlying haemodynamic profile of a given patient or population with PH-LHD including acceleration time, velocity time integral (VTI) and presence/absence/timing of systolic notching.21,23 These right heart metrics should be evaluated in conjunction with standard left heart metrics, including LA size, estimated LA pressure (by mitral inflow and tissue Doppler assessment), LV size and function, and valvular dysfunction, which in turn can then aid in distinguishing IpcPH and CpcPH.16
The ratio of TAPSE/PASP has been described as an index of right ventriculo-arterial coupling independent of LV dysfunction, and has been validated with invasive haemodynamics by Tello et al.24 Gerges et al. demonstrated this as being valuable in being able to differentiate between IpcPH and CpcPH in the setting of both HFrEF and HFpEF.25 Guazzi et al. showed it could be used to prognosticate in HFpEF patients, with higher TAPSE/PASP correlating with higher levels of natriuretic peptides, worse systemic and pulmonary haemodynamics and abnormal exercise aerobic capacity.26
With recent attention to PAC across the PH spectrum, including in PH-LHD, with increased pulsatile load (secondary to elevated PAWP) reducing PAC, we have described a non-invasive surrogate for PAC using the RVOT–VTI/PASP relationship, which we showed stratifies patients with IpcPH and CpcPH as compared with pulmonary arterial hypertension (PAH), and correlated with the 6-minute walk distance.14,27–29
Right Heart Catheterisation
In patients with suspected PH-LHD, right heart catheterisation (RHC) is required to prove the diagnosis and to differentiate between pre-capillary PH (PAH) and PH-LHD and to further distinguish IpcPH and CpcPH. Although the procedure is relatively safe and is now routine practice in most centres, there is a hesitancy to apply this as routine in all PH-LHD patients, given its invasive nature and potential for misinterpretation of the data. Our recommendation is that RHC should be performed in the following circumstances:
- diagnostic uncertainty based on noninvasive testing;
- disproportionate symptoms compared with echocardiographic findings;
- progressive symptoms despite optimal medical therapy;
- when advanced therapies are planned especially transplantation or mechanical circulatory support.
One major drawback with RHC in this patient population is that the pivotal measurement, PAWP, is the most prone to errors and extra time and care should be taken while documenting PAWP. To minimise this error, the reference level needs to be at the mid-thoracic position and the catheter tip position should be verified (with either fluoroscopy and with aspiration and assessment of PAWP blood) and the PAWP should be measured at the end of the expiratory phase of normal respiration to minimise respirophasic variations.30,31 If there are still concerns about the accuracy of the PAWP measurement, then direct measurement of the left ventricular end-diastolic pressure (LVEDP) can be performed. However it must be remembered that LVEDP is a measure of LV preload and LV diastolic compliance, and this is not a true surrogate for PAWP, which is both the best reflection of the total effect of LHD on the pulmonary circulation and has been shown to be a better predictor of outcomes, especially in the HFpEF population.32–34
In addition to standard measurements, other procedural techniques may be required in patients with PH-LHD. These patients are frequently on diuretic therapy, which can lead to artificially lower PAWP measurements than are normal for the patient; in this case a 500 cc IV fluid challenge can be performed with reassessment of haemodynamic measurements. This can be especially helpful in patients with HFpEF, where there can be vast differences in haemodynamics based on volume status.35 Also, testing during exercise is important in this population as it is both required to diagnose or confirm HFpEF, especially if resting PAWP is <15 mmHg, and it is a useful in ‘unmasking’ exercise-induced PH where there may be a disproportionate rise in mPAP in relation to changes in cardiac output.36
Management
Optimising Goal-directed Therapy
The main goal of management in this population should be optimisation of underlying medical therapies, using device therapy and addressing underlying valvular disease where indicated. In particular, the use of adequate diuretic therapy, an often under-emphasised avenue of therapy, is vital for symptom control. The CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association functional Class III Heart Failure Patients (CHAMPION) trial showed that invasive monitoring of left-sided filling pressures using the pulmonary artery diastolic pressure (as a surrogate marker of PAWP) to guide diuretic therapy reduces HF hospitalisations in a homogenous HF population.37 This study has led to interest in the potential role of this form of monitor-guided diuretic therapy in PH-LHD and upcoming studies using the CardioMEMS device, such as the Hemodynamic-GUIDEd Management of Heart Failure trial (NCT03387813), may provide more evidence for its use.
While there have been recent advances in the medical therapies of HFrEF using two new agents – angiotensin receptor neprilysin inhibitors and sodium–glucose cotransporter 2 inhibitors – these have not been specifically evaluated in PH-LHD. There is interest in the effect of these drugs on cardiopulmonary haemodynamics, with active trials enrolling for both drug classes – Pulmonary Artery Pressure Reduction with ENTresto (Sacubitril/Valsartan) (PARENT; NCT02788656) and Empagliflozin Impact on Hemodynamics in Patients With Heart Failure (EMBRACE-HF; NCT03030222). To date, there remains no specific therapy for HFpEF; however, there is promising data in the use of the interatrial shunt device, although this study and the pivotal trial have excluded patients with PVR >4 WU and therefore there is uncertainty as to its transferability to the broader PH-LHD population.38
Pulmonary Hypertension-specific Therapy
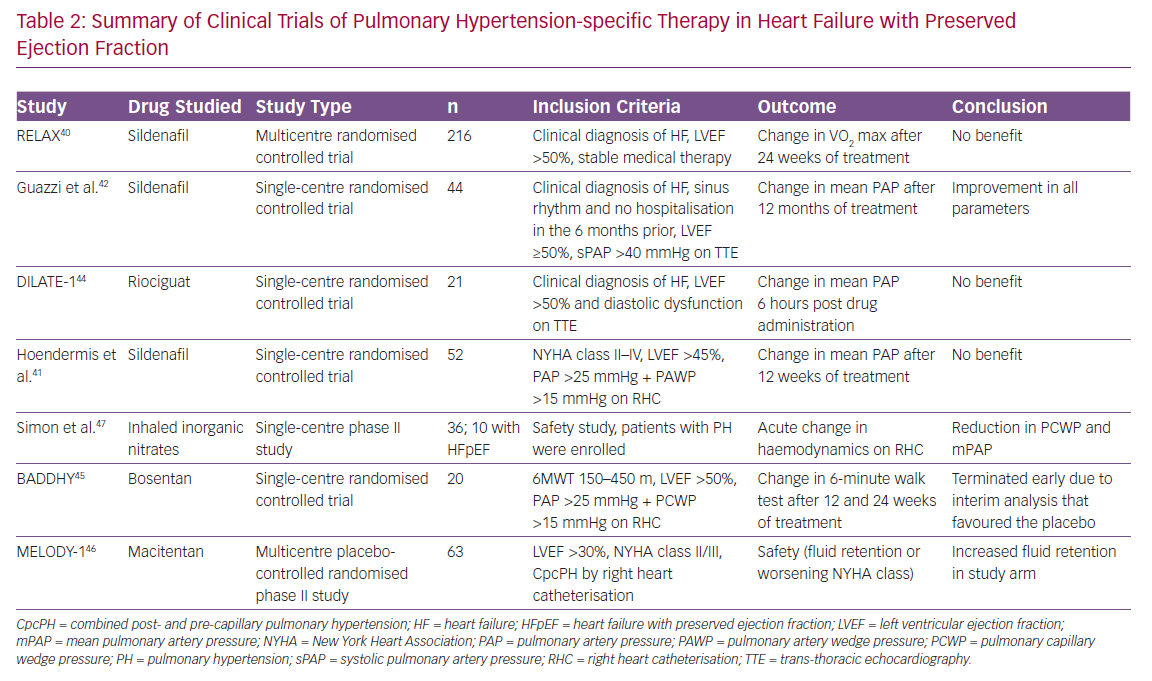
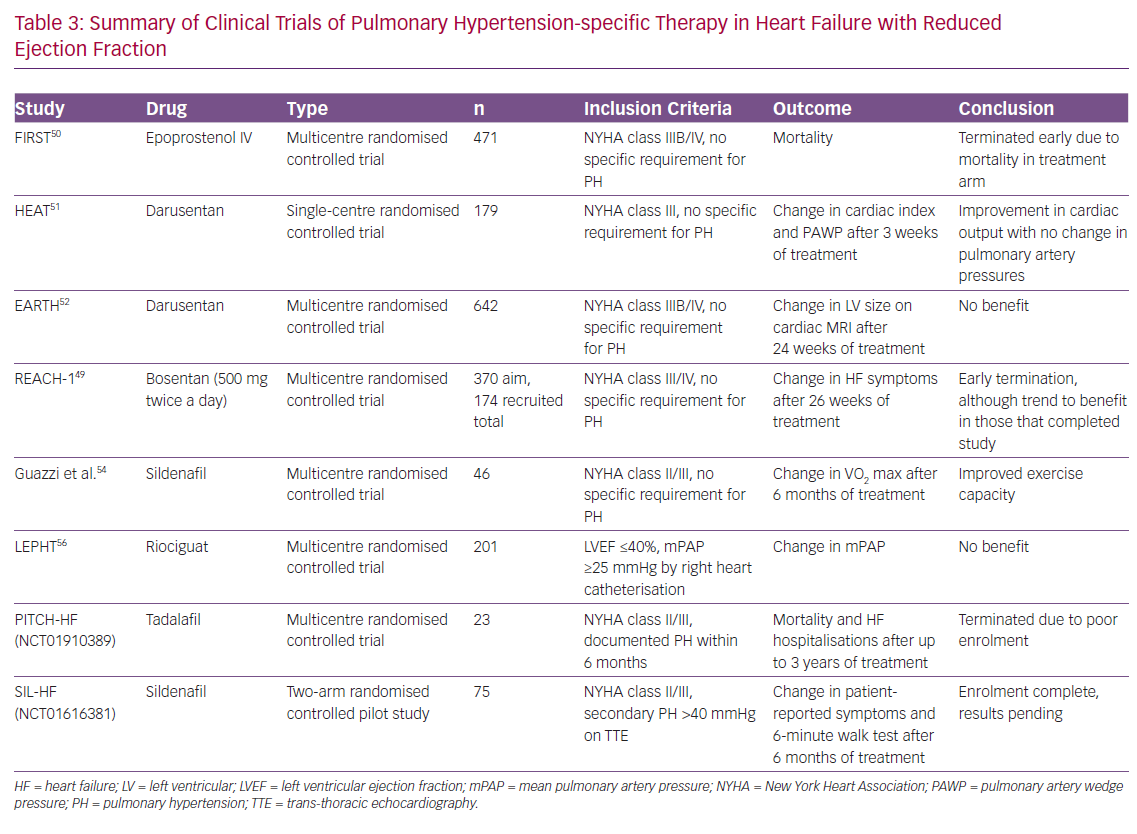
As PAH and PH-LHD share a number of common pathophysiological pathways and neurohumoral perturbations, there have been a number of studies performed to assess the efficacy of PH-specific therapy in the PH-LHD population.39 In general, given lack of positive trial data along with the potential increased risk of pulmonary oedema in the setting of improved trans-pulmonary flow, the use of PH-specific therapy is not recommended. We have summarised these studies in Tables 2 and 3.
Heart Failure with Preserved Ejection Fraction
Given the paucity of other treatment options for heart failure with preserved ejection fraction, several studies have been undertaken in this population. The largest study has been the Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure (RELAX) trial, which enrolled patients with HFpEF and assessed the effect of sildenafil, a PDE5 inhibitor, on the clinical endpoint of exercise tolerance and clinical status.40 This trial was negative and while showing the lack of benefit of sildenafil in the broader HFpEF population, it is important to note that this trial did not specifically study patients with PH-LHD, nor did it assess the effects on pulmonary haemodynamics.
Another smaller study performed by Hoendermis et al. evaluated the haemodynamic effects of sildenafil versus placebo in PH-HFpEF, but this was also negative.41 The authors note that this study evaluated largely IpcPH (median PVR ~2.6 WU), which comprised 65% of the study population, and relatively mild PVR elevation in those with CpcPH (median PVR 4 [IQR 3.4–4.8]). Conversely, Guazzi et al. conducted a study evaluating the role of sildenafil in a randomised, placebo-controlled trial of 44 patients with PH-HFpEF (largely CpcPH; mean PVR 3.6 WU) and found sustained haemodynamic benefits and improvements in RV size and function in the sildenafil group.42 More recently, Bermejo et al. found worse outcomes after long-term sildenafil use compared with placebo in patients’ status after corrective valvular surgery.43 Furthermore, riociguat, a nitric oxide pathway soluble guanylate cyclase stimulator, was studied in the Acute hemodynamic effects of riociguat in patients with PH associated with diastolic heart failure (DILATE-1) study and showed that, while there was safety using this medication, there was no significant benefit with regards to haemodynamic endpoints.44
The Safety and Efficacy of Bosentan in Patients With Diastolic Heart Failure and Secondary Pulmonary Hypertension (BADDHY) trial used bosentan, a dual endothelin A and B antagonist.45 It had to be prematurely halted as there was a trend to harm in the treatment arm. Macitentan in PH due to left ventricular dysfunction (MELODY-1) was a phase II trial studying macitentan, a dual endothelin A and B antagonist, in CpcPH due to either HFpEF or HFrEF (although overwhelmingly a HFpEF population), and showed increased fluid retention in the treatment arm within 4 weeks of initiating therapy and worsening functional class without an improvement in haemodynamic variables and a non-significant decrease in NT-proBNP in the macitentan arm.46 Finally, organic nitrates as a direct activator of the nitric oxide pathway has been investigated as a novel therapeutic area. Simon et al. conducted a phase II dosing clinical trial that demonstrated a reduction in PAWP and PA pressures from inhaled nitric oxide, and this effect was greater in HFpEF patients compared with those with PH alone.47
There are currently a number of ongoing trials in PH-HFpEF patients, including the Hemodynamic Evaluation of Levosimendan in Patients With PH-HFpEF (HELP) study (NCT03541603) evaluating levosimendan, a calcium sensitiser with inotropic, lusitropic and vasodilatory properties; a randomised, placebo controlled trial of organic nitrites, Oral Nitrite in Patients With Pulmonary Hypertension and Heart Failure With Preserved Ejection Fraction (NCT03015402); and a phase II clinical trial of metformin – Metformin for PH HFpEF (NCT03629340) – among others.
Heart Failure with Reduced Ejection Fraction
A number of trials have been performed in the broader HFrEF population, but at this stage data are lacking to support the use of PH-specific therapy. Initial clinic trials using bosentan, IV prostacyclins and darusentan (a selective endothelin AT antagonists) were all negative.48–52 A major criticism of these studies is that they failed to focus on the PH-LHD population and often had higher doses of these therapies than used in the PAH population.
More focused studies have been performed to assess the potential efficacy of sildenafil. In a single arm, open-label study, Lewis et al. showed a significant improvement in haemodynamics and cardiopulmonary exercise testing parameters (including VO2 max and increase in ventilation with respect to CO2 output) with 50 mg sildenafil, while Guazzi et al. performed a single-centre, randomised trial that showed improvements in haemodynamics, echocardiographic markers of left ventricular diastolic function and cardiac geometry, as well as functional status (by CPET) and quality of life.53–55 However, double-blind, placebo-controlled trials with PDE5 inhibitors have been plagued by poor recruitment. The Phosphodiesterase Type 5 Inhibition With Tadalafil Changes Outcomes in Heart Failure (PITCH-HF; NCT01910389), evaluating tadalafil, was terminated after funding was withdrawn due to a number of factors, including poor enrolment.
There have been two other recently published studies using PH-specific therapy. The Study to Test the Effects of Riociguat in Patients With Pulmonary Hypertension Associated With Left Ventricular Systolic Dysfunction (LEPHT) evaluating riociguat failed to show a reduction in PAP or PVR after 16 weeks of treatment.56 The Study to Evaluate Whether Macitentan is an Effective and Safe Treatment for Patients With Heart Failure With Preserved Ejection Fraction and Pulmonary Vascular Disease (SERENADE; NCT03153111) trial is a phase IIb trial which is currently underway.
Left Ventricular Assist Device
Left ventricular assist device (LVAD) therapy has become a mainstay in the treatment of end-stage HFrEF, with multiple devices now FDA approved for both bridge-to-transplant (BTT) and destination therapy (DT).57 Many studies have shown reversal of PH-LHD with LVAD support causing both mechanical unloading of the left ventricle, and the persistent reductions in filling pressures leading to reverse remodelling of the pulmonary vasculature changes in CpcPH. This has been shown in a number of single-centre observational studies in the pre-transplant population and in a more recent study which showed significant reduction in PH when compared with medical therapy in a similar population.58–62
However, there is a subgroup that has persistent CpcPH after LVAD implantation and there is no consensus on treatment for this group. There have been several small trials evaluating the role of sildenafil after LVAD placement. In a single-centre study, Tedford et al. showed sildenafil treatment led to a significant reduction in mPAP, improved cardiac output and reduction in PVR in LVAD patients with residual elevated pulmonary pressures more than 1-month post implant.63 Other agents, including bosentan, have been evaluated.64 The Clinical Study to Assess the Efficacy and Safety of Macitentan in Patients With Pulmonary Hypertension After Left Ventricular Assist Device Implantation (SOPRANO; NCT02554903) study is ongoing.
Thus, while the data suggest that LVAD therapy is associated with improvements in cardiopulmonary haemodynamics acutely and over time, there are patients who have persistent PH and/or RV failure (early or late) after LVAD implantation. While several smaller trials suggest haemodynamic benefit from the use of PH-specific therapy, and we use such therapy in isolated cases, there is currently a lack of large randomised data to support its use more broadly across this population.
Transplantation
Orthotropic heart transplantation (OHT) is still considered the definitive treatment for end-stage HFrEF. Unfortunately, patients with PH-LHD have worse outcomes post-transplantation, specifically those patients with a PVR >2.5 WU who do not demonstrate reversibility with vasodilator challenge, have significantly higher risk of mortality due to RV failure at 3 months (33%; 14% related to RV failure versus 6%).65 This was further shown in an analysis of the United Network for Organ Sharing (registry that showed pre-transplant PVR >2.5 WU was an independent predictor of mortality), although the degree of elevation of PVR modestly increased mortality in a non-linear manner.66
These studies demonstrate that the evaluation of PH-LHD in the context of OHT must be both dynamic and repeated, and that a stepwise approach to the transplant candidate with an elevated PVR is vital in patients where the PVR remains elevated. Without a viable mechanical support option, as may be the case in the congenital population, selected patients may be eligible for combined heart–lung transplantation. This option, however, is not without significant pitfalls, because this procedure is performed at only a select number of centres and has a high post-operative morbidity and mortality when compared with OHT.67
Conclusion
PH-LHD is a major problem for patients with both HFrEF and HFpEF and limited targeted treatment options have proven beneficial for this population. Although trials to this date have been negative, the combination of more nuanced phenotyping of this patient population combined with novel modalities is providing hope of advances in treatment.
A corrigendum has been published for this article: https://doi.org/10.15420/cfr.2020.1.1